- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Soft condensed matter
- Functionalisation of highly flexible metal organic framework materials
Functionalisation of highly flexible metal organic framework materials
Crystalline porous hybrid solids or metal organic frameworks (MOFs) are of interest due to their potential applications in domains such as separation, gas storage, catalysis or biomedicine. While MOFs are usually rigid structures with large unit cells, some of them exhibit a very large and reversible swelling under external stimuli including pressure, temperature, light, gas or solvent adsorption. The overall performance of the flexible MOF depends on the structure type and the nature of the constitutive metal and linker [1]. MOFs are usually only available as powders. Nonetheless, high resolution structural data can be obtained using microdiffraction techniques. In this work single-crystal microdiffraction was performed at beamline ID13 with about a 1 μm beam.
We have previously shown [2] that these solids possess a giant and reversible increase in their cell volumes (~85-220%) upon solvent sorption and also possess a selective sorption capacity while retaining their crystal structure. Replacement of one or several aromatic proton(s) by functional groups strongly affects the flexible character of these phases. We report here how the swelling of a series of iron(III) dicarboxylates, denoted MIL-88B or D, built from a hexagonal array of trimeric iron(III) building units and dicarboxylate moieties, can be tuned by changing the functional groups grafted onto the organic spacer.
 |
|
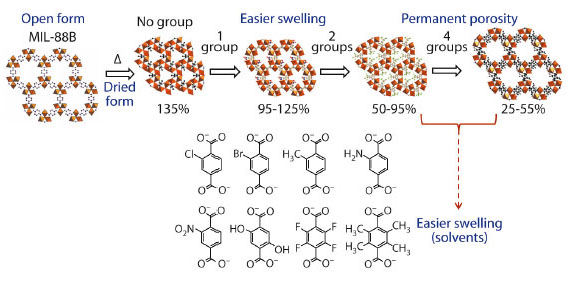
Fig. 92: Schematic representation of the impact of functional groups over the porosity and the swelling behaviour of the iron(III) terephthalate MIL-88B(Fe). |
The effect of the modifications was dependent on the nature, size and number of functional groups grafted onto the organic spacer (Figure 92) [2]. The initial degree of pore opening of the dried solid can be varied. This allows the amplitude of the swelling to be varied and can lead to either dried solids being non porous or possessing a permanent porosity to nitrogen (at -196°C). As a consequence, the increase in pore opening is associated with a lower degree of interactions between the aromatic spacer units from the dried structure, and the adsorption of solvents becomes much easier. In addition, the presence of polar or apolar functional groups allows the sorption selectivity of the porous solid to be tuned. This paves the way for the use of functionalised flexible MOFs for separation or drug delivery applications
Principal publication and authors
P. Horcajada (a), F. Salles (b), S. Wuttke (a,c), T. Devic (a), D. Heurtaux (a), G. Maurin (b), A. Vimont (c), M. Daturi (c), O. David (a), E. Magnier (a), N. Stock(d), Y. Filinchuk (e,f), D. Popov (g), C. Riekel (h), G. Férey (a) and C. Serre (a), J. Am. Chem. Soc. 133, 17839 (2011).
(a) Institut Lavoisier, (UMR CNRS 8180), Université de Versailles (France)
(b) Institut C. Gerhardt Montpellier, UMR CNRS 5253, ENSCM, Montpellier (France)
(c) Laboratoire Catalyse et Spectrochimie LCS, UMR CNRS 6506, ENSICAEN, Caen (France)
(d) Institute of Inorganic Chemistry, Christian-Albrechts-Universität, Kiel (Germany)
(e) SNBL at ESRF, Grenoble (France)
(f) Institute of Condensed Matter and Nanosciences, Université Catholique de Louvain, Louvain-la-Neuve (Belgium)
(g) HPCAT, Geophysical Laboratory, Carnegie Institution of Washington, Argonne (USA) (h) ESRF
References
[1] G. Férey and C. Serre, Chem. Soc. Rev. 38, 1380 (2009).
[2] C. Serre, C. Mellot-Draznieks, S. Surblé N. Audebrand, Y. Filinchuk and G. Férey, Science 315, 1828 (2007).



