- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Structure of materials
- Fast time-resolved cement hydration studies
Fast time-resolved cement hydration studies
Modern architecture cannot get along without concrete, no matter whether it is a giant skyscraper or an underground project. Concrete is made from sand, gravel, additives, water, and cement. Cement is the component in concrete that holds the other components together. To control the properties of concrete, it is necessary to know what occurs as it hardens. We have studied the first seconds in this process, the first few seconds in the “life of a building”, by means of X-ray diffraction. Furthermore, these studies made it possible for us to understand the role of superplasticisers added to the initial cement water mixture.
Ordinary Portland cement (OPC) is a mixture of finely ground limestone, clay, sand, calcium silicates, aluminum and iron compounds and sulfates. Directly after mixing with water, chemical reactions between the components of cement and water lead to solidification and hardening. The rapidly initiated reaction of the cement clinker component C3A (Ca3Al2O6) with sulfate (SO42–) to form ettringite (Ca6Al2(SO4)3(OH)12·26H2O) is one of the important steps [1]. The enormous stability of concrete stems from crystalline needles of ettringite that are formed during this process. These needles are firmly interlocked with each other.
Different additives are used to optimise the properties of concrete. These additives improve the flow of the concrete, making it easier to process. Furthermore, the water content can be reduced which improves the compressive strength of concrete. A typical class of superplasticisers is based on polycarboxylate-ether (PCE) [2].
 |
|
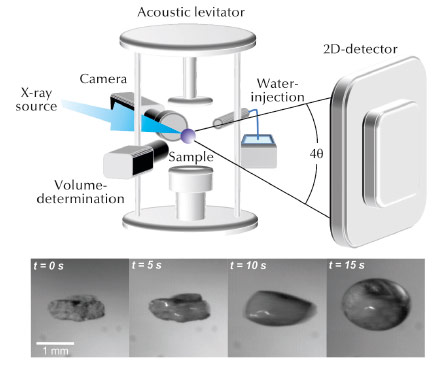
Fig. 130: Contact free analysis of the hydration of cement using an acoustic levitator. |
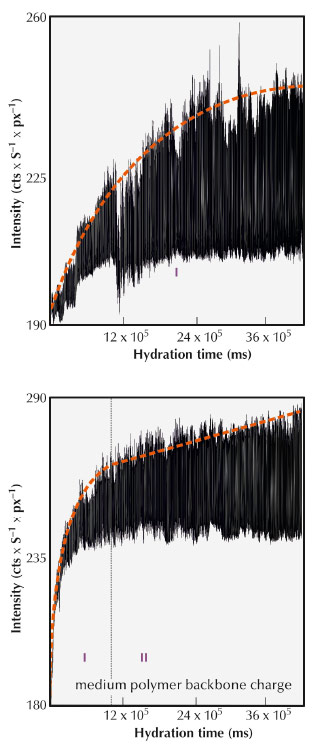
The formation of the phases at the beginning of the hydration process was not understood in detail. Detailed insight into the different stages of the hydration process is essential for a more complete understanding of how these processes can be effectively influenced. Time resolved in situ X-ray diffraction experiments with a resolution of 500 ms were carried out at beamline ID11 to follow this reaction. The reaction was carried out in an acoustic levitator (see Figure 130). A piece of Portland cement was held in suspension by acoustic waves in this sample environment. The reaction was initiated by spraying a defined amount of water onto the levitated sample. The experiment was repeated with samples containing different amounts of PCE superplasticisers. To characterise the influence of the superplasticisers, the development of the ettringite reflection (100) was investigated in detail. The hydration behaviour of the cement specimen is driven by the anionic charge densities of the PCE. The C3A in pure OPC reacts to ettringite as a function of the residual sulfate in the pore solution. This hydration behaviour results in a high initial formation of ettringite and its amount increases as a function of time (see Figure 131). The ettringite formation in PCE containing OPC is additionally influenced by the backbone charge density of the respective PCE. During the reaction with water, PCE is replaced by sulfate ions from the pore solution. An equilibrium between PCE adsorption and its replacement by SO42– anions leads to a linear increase in the amount of ettringite. Consequently, a higher polymer backbone charge leads to a retarded adjustment of the equilibration state. Fewer sulfate ions replace PCE and the formation of ettringite is decelerated. Immediately after the contact between water and cement, the PCE adsorbs onto the surface of the clinker C3A. The particles, sterically stabilised in this way, remain in suspension and the PCE is then gradually replaced by sulfate ions, which retards the incipient ettringite crystallisation. Consequently, more free water is left in the system, dissolving more crystalline components and the resulting concrete can thus flow for a longer period and becomes more dense.
 |
|
Fig. 131: Side view of the ettringite (100) reflection as a function of time. Top: Ordinary Portland cement; bottom: OPC with PCE admixtures. The growth process can be separated into an initial exponential (I) and into a linear (II) increase of the reflection intensity (dashed line). |
Principal publication and authors
M.-C. Schlegel, A. Sarfraz, U. Mueller, U. Panne and F. Emmerling, Angew. Chem. Int. Ed. 51, 4993–4996 (2012).
BAM Federal Institute of Materials Research and Testing, Berlin (Germany)
References
[1] A.N. Christensen, T.R. Jensen and J.C. Hanson, J.Solid State Chem. 177, 1944–1951 (2004).
[2] R.J. Flatt, I. Schober, E. Raphael, C. Plassard and E. Lesniewska, Langmuir 25, 845–855 (2009).



