- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Structure of materials
- From discovery to invention – rational design of zeolites
From discovery to invention – rational design of zeolites
Since their discovery, zeolites have become the heavy duty molecular machinery of the chemical industry. With their unique porous frameworks, these crystalline aluminosilicates are essential as catalysts and adsorbents for a wide range of applications in petrochemistry, environmental chemistry and water treatment. Despite their importance, the fraction of zeolites used in commercial applications represents less than 10% of all zeolite frameworks hitherto described. This contrast originates from the fact that new zeolites, up to now, have either been discovered in geological deposits or in trial and error syntheses. Until now, rational design of new frameworks to fulfil a specific function in chemical processes has been out of reach. However, the need for reconversion of our fossil fuel based economy makes the availability of zeolites with a tailored framework structure and functionality more important than ever.
Currently, zeolite synthesis exploits mono-, oligo- and polymeric sources of framework elements. The resulting zeolite topologies depend on a large number of parameters and the outcome of most syntheses remains unpredictable. Envisioning rational zeolite design, pre-fabricated building units have been proposed as an alternative to monomeric or colloidal species. Re-assembly of building units existing in a known zeolite provides a promising route. Such building units can for example be harvested by controlled disassembly of existing zeolites. Upon isolation, the building units obtained can be rearranged. Essentially, this comes down to the transformation of existing zeolites into new frameworks with tailored properties.
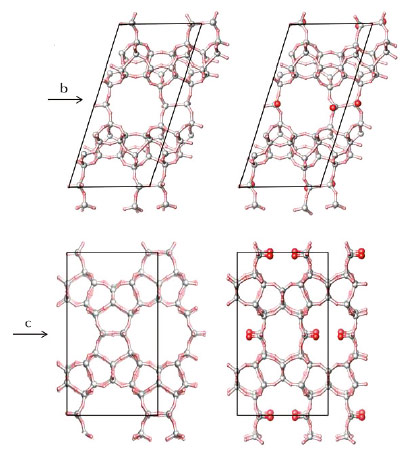
Recently, two new zeolites COK-14 and –COK-14 were created via this approach. COK-14 is the first material with a new all-silica framework topology (OKO framework) with a two-dimensional channel system and interconnecting 8-, 10- and 12-membered rings (Figure 137). In its interrupted form, called –COK-14, the zeolite additionally has silanol groups pointing systematically into the 12-membered ring (Figure 137). To synthesise these zeolites, building units from the existing IM-12 zeolite were harvested and selectively rearranged. The structure of IM-12 zeolite consists of all-silica sheets interconnected by cubic struts containing one germanate and one silicate four-ring (4R). Acid treatment selectively removes the germanate 4R from the framework leaving the silicate 4R attached to the intact silicate sheets. This structure is consequently recycled as a building unit and reconnected into COK-14 or –COK-14, depending on the hydration state. Overall, the transformation from IM-12 to COK-14 involves the removal of one layer of T-atoms and as such is the first experimentally observed inverse sigma transformation.
 |
|
Fig. 137: COK-14 framework (left) and -COK-14 framework (right). OH groups in –COK-14 are highlighted in red. |
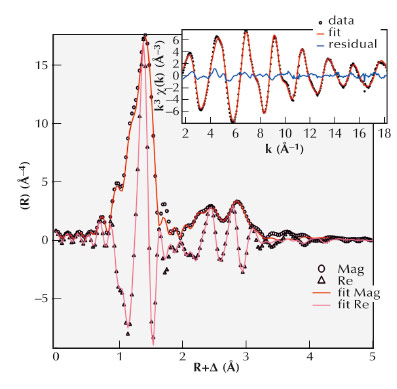
The choice for IM-12 zeolite as a source of building units resulted from its interesting geometry of silicate layers connected by germanate and silicate four-rings. The arrangement of germanium (Ge) in this zeolite was analysed by Ge K-edge EXAFS at BM01B, the Swiss Norwegian Beamline (SNBL), in collaboration with BM26, DUBBLE Beamline. EXAFS analysis revealed that each Ge centre was connected to 4 oxygen atoms (3+1) at respectively 1.75 Å and 1.85 Å (Figure 138) and two Ge neighbours at 3.16 Å. In combination with high resolution powder X-ray diffraction (HRXRD) data obtained from the same sample, this geometric unit was localised unambiguously as a germanate 4R in the cubic unit connecting all-silicate layers in the structure of IM-12.
 |
|
Fig. 138: k3 weighted Ge K-edge EXAFS data as inset and its Fourier transform (magnitude/real part) for IM-12. |
Ge K-edge EXAFS and HRXRD measurements were of key importance to reveal the Ge distribution in IM-12. The presence of a Ge four-ring in the structure allowed us to isolate separate silicate building units. These can be alternatively stacked to form new zeolites in a more rational zeolite synthesis approach. Discovering the ordered arrangement of Ge in IM-12 zeolite not only led to the synthesis of new zeolites –COK-14 and COK-14. With this knowledge, we are now studying the synergy between the overall zeolite synthesis conditions and the final Ge arrangement in the framework. This leads to several new synthesis approaches for obtaining similar Ge distributions in a zeolite.
Principal publication and authors
E. Verheyen (a), L. Joos (b), K. Van Havenbergh (c), E. Breynaert (a), N. Kasian (a, d), E. Gobechiya (a), K. Houthoofd (a), C. Martineau (e), M. Hinterstein (f), F. Taulelle (e), V. Van Speybroeck (b), M. Waroquier (b), S. Bals (c), G. Van Tendeloo (c), C. E.A. Kirschhock (a) and J.A. Martens (a), Nat. Mater. 11, 1059-1064 (2012).
(a) Center for Surface Chemistry and Catalysis, KU Leuven, Heverlee (Belgium)
(b) Center for Molecular Modeling, Ghent University, Zwijnaarde (Belgium)
(c) Electron Microscopy for Materials Science, University of Antwerp (Belgium)
(d) L.V. Pisarzhevsky Institute of Physical Chemistry, National Academy of Sciences of Ukraine, Kyiv (Ukraine)
(e) Tectospin, Institut Lavoisier, UMR 8180 Université de Versailles Saint Quentin en Yvelines (France)
(f) Institut für Werkstoffwissenschaft, Technische Universität Dresden (Germany)



