- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structural biology
- Shaping the early event of prion formation
Shaping the early event of prion formation
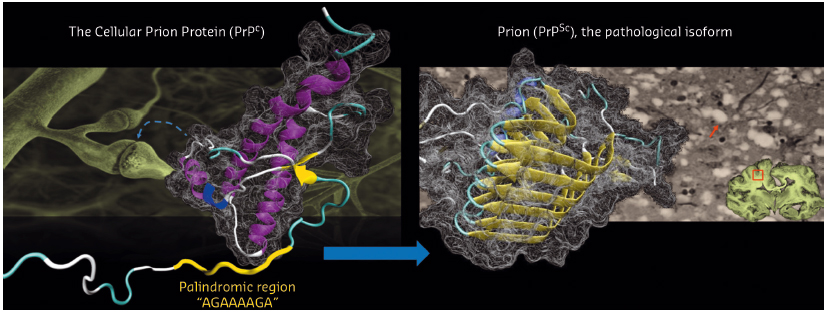
The conversion of the cellular prion protein (PrPC) into its misfolded and amyloidogenic isoform, denoted as prion or PrPSc, is the central event in prion diseases which affect humans and animals. In prion biology, unravelling the molecular mechanisms leading the conversion process whereby α-helical motifs are replaced by β-sheet secondary structures is of utmost importance.
 |
|
Fig. 116: Structural conversion of the cellular prion protein (PrPC) to its pathological isoform, denoted as prion or PrPSc. PrPC is mostly involved in synaptic functions. PrPSc causes prion diseases such as Creutzfeldt-Jakob disease in humans which is characterised by spongiform neurodegeneration (arrow) in the brain. |
The structure of human PrPC consists of a disordered N-terminal part (residue 23-127) and a structured C-terminal domain (residue 128-228) (Figure 116). The insoluble and heterogeneous nature of PrPSc makes its structural characterization extremely difficult. The N-terminal region of PrPC features a palindromic sequence (AGAAAAGA) which can lead to the formation of neurotoxic fibrils enriched in β-sheet motifs. Experimental evidence also supports the idea that the palindromic sequence plays a critical role for prion generation and transmissibility. However, the intrinsic flexibility of this N-terminal region has hampered efforts to obtain atomic information on the structural features of the palindromic sequence.
In this study, we used nanobodies (Nb) to solve the first structures of the full-length HuPrP and its C-terminal truncated version by X-ray crystallography. Nb are small (15 kDa) and stable single-domain fragments harbouring the full antigen-binding capacity of the original heavy chain–only antibodies that naturally occur in camelids. Collective efforts of several laboratories have demonstrated that Nbs are exquisite chaperones for crystallising complex biological systems such as membrane proteins, transient multiprotein assemblies, transient conformational states and intrinsically disordered proteins [1].
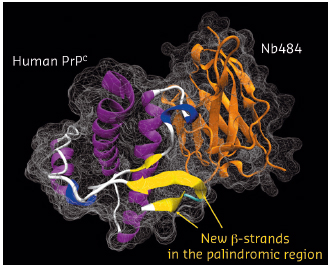
In complex with this Nb, the total amount of structured polypeptide (125 amino acids of antibody and 108 amino acids of the prion protein) rises to 71% as against 52% for free HuPrP, thus providing a much better starting point for crystallisation. The high-resolution X-ray crystal structures (obtained using data collected at beam-line BM30A) of two HuPrPs in complex with a selective nanobody (Nb484) revealed a novel structural feature. While the segment from residues 128 to 225 shares a fold that is very similar to the corresponding NMR HuPrP structures, the binding of Nb484 to a region adjacent to the first β-sheet (β1) unveils key structural features of the hydrophobic segment from residues 117 to 128, which had remained unresolved in all the PrP structures published so far.
 |
|
Fig. 117: Crystal structure of the human PrPC in complex with a nanobody (Nb484) with the newly formed β-strands in the palindromic region highlighted. |
In our X-ray structures, the sequence including the palindromic motif arranges in a novel β-strand we denoted as β0 (residues 118–122), which folds into a three-stranded antiparallel β-sheet with β1 and β2 (Figure 117). The same structural arrangement was observed in both crystal structures, suggesting that it does not result from crystal packing but might have major biological implications for prion conversion. Edges of regular β-sheets are inherently aggregation-prone because the motif for H-bonding with any other β-strand is available. From our structures, it appears that the exposed edge of the short β1 strand observed in all previous PrP structures serves as an intra-molecular nucleus for edge-to-edge β aggregation of part of the palindromic sequence to form the β0 strand. The propensity of the palindromic sequence to engage in such β-structures strongly indicates that this motif mediates β-enrichment in the PrPC monomer as one of the early events in the PrPC to PrPSc conversion.
Here, we show that Nb-assisted crystallography is a powerful tool for unveiling local structural features of intrinsically disordered proteins. Our data provide structural evidence that the palindromic motif of HuPrPs is important as a dynamic site for β-sheet structural conversion in prion formation. The structures we solved also support the hypothesis that the conserved palindromic sequence mediates β-enrichment in the PrPC monomers as one of the early events in prion formation. Moreover, crystals of the full-length human PrP in complex with Nb484 are amenable to soaking experiments to study the interactions of small molecules with the flexible region of HuPrP [1].
Principal publication and authors
R.N.N. Abskharon (a, b, c), G. Giachin (d, e), A. Wohlkonig (a, b), S.H. Soror (a, b, f), E. Pardon (a, b), G. Legname (d) and J. Steyaert (a, b), J Am. Chem. Soc. 136, 937-944 (2014).
(a) Structural Biology Brussels, Vrije Universiteit Brussel (Belgium)
(b) Structural Biology Research Center, VIB, Brussels (Belgium)
(c) National Institute of Oceanography and Fisheries (NIOF), Cairo (Egypt)
(d) Department of Neuroscience, Laboratory of Prion Biology, Scuola Internazionale Superiore di Studi Avanzati (SISSA), Trieste (Italy)
(e) Present address: ESRF.
(f) Center of Excellence, Helwan Structure Biology Research, Faculty of Pharmacy, Helwan University, Cairo (Egypt)
References
[1] E. Pardon et al., Nat Protoc 9, 674-93 (2014).



