- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Soft condensed matter
- Skeletal cubic, lamellar, and ribbon phases of bundled thermotropic bolapolyphiles
Skeletal cubic, lamellar, and ribbon phases of bundled thermotropic bolapolyphiles
Ordered periodic structures can self-assemble on several different length scales. Crystals form by ordered packing of atoms, i.e. units on the 0.1 nm scale. Block copolymers can form regular lattices of the different polymer blocks typically of 10-100 nm size and colloidal particles, such as polystyrene beads or inorganic glassy spheres (such as in opal), can form “colloidal crystals” with periodicities between a fraction of a µm to several µm. The most prominent periodic self-assembled systems in the 1-10 nm range can be classified as liquid crystals (LC) in a broader sense. They contain molecules typically combining two or more poorly compatible chemical groups (amphiphilic or polyphilic) as well as combining both flexible and rigid units of rod-, disk-, or wedge-like shape. This expanding field of soft matter science has produced some very intriguing structures, growing in complexity year after year. The challenge is to learn how to design molecules that will assemble into pre-determined superstructures, which could be used in molecular electronic and optical devices, sensors, actuators, membranes and other smart molecular materials.
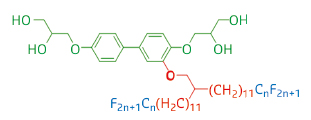
In the work highlighted here, a series of T-shaped polyphilic molecules composed of a rigid linear aromatic core with a polar glycerol group at each end and one “swallow-tail” partially fluorinated side-chain of different lengths n (see Figure 39) were synthesised and their LC phases were investigated by small-angle grazing incidence X-ray scattering (GISAXS) at beamline BM28, as well as by other techniques.
 |
|
Fig. 39: Structure of the T-shaped polyphilic molecules. |
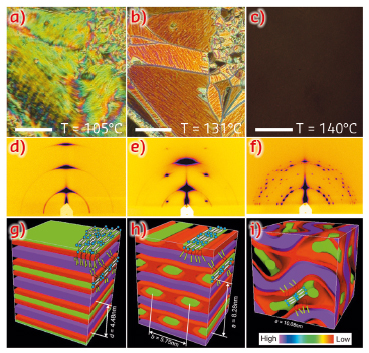
Similar molecules with smaller side-groups show a remarkable series of honeycomb-like LC phases with the aromatic rods as the “wax” and the side-chains as “honey”. However, here we find that, as the side-groups increase in size, the honeycomb cells open up, resulting in a structure of alternating rigid aromatic and flexible fluoroalkyl layers (Figure 40g). As temperature is raised, the flexible side-chains expand faster than the rigid cores, and the growing mismatch results in the aromatic layers (green) being shredded into parallel ribbons (symmetry c2mm). Interestingly, the molecules are highly aligned along the ribbon axis, forming a nematic LC phase self-confined within these 2-3 nm wide channels (Figure 40h).
 |
|
Fig. 40: (a-c) Texture as seen between crossed polarisers in the (a) lamellar phase at 105°C; (b) ribbon phase at 131°C and (c) Cubic Ia3d phase at 140°C. Scale bar: 50 µm. (d-f): GISAXS patterns from a thin film in the (d) lamellar, (e) ribbon and (f) cubic phase. (g-i): Histogrammatic electron density maps with added schematic molecules for (g) lamellar, (h) ribbon, and (i) cubic phase. Discrete solid colours are allocated to three different density ranges, purple for the highest, green for the middle and red for the lowest; they correspond roughly to chemical group colours in the chemical formula. |
As the temperature is increased further, birefringence is lost (Figure 40c) as the structure becomes cubic (Ia3d, Figure 39). This phase is of the cubic type known as “double gyroid” bicontinuous, also found in some lipid-water systems, block copolymers and some LC systems. It consists of two infinite interpenetrating networks that never touch. However, unlike in any other bicontinuous phase, the molecular rods are parallel to the segments of the networks. Also, the distance between the network junctions is exactly two molecular lengths and is fixed.
This new mode of molecular self-assembly promises potential 3-D semiconductors for applications such as organic photovoltaic materials that need no alignment. For alignment is often hard to achieve and is required in the alternative anisotropic systems such as columnar LCs. This work also shows how a single appropriately designed organic compound can display molecular layers, wires and networks just by changing temperature. If required, each of these could be stabilised and fixed by chemical cross-linking.
Principal publication and authors
F. Liu (a), M. Prehm (b), X.B. Zeng (a), C. Tschierske (b) and G. Ungar (a), J. Am. Chem. Soc. 136, 6846−6849 (2014).
(a) Department of Materials Science and Engineering, University of Sheffield (UK)
(b) Institute of Chemistry, Organic Chemistry, Martin-Luther-University Halle-Wittenberg, Halle (Germany)



