- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Complex systems and biomedical sciences
- Small angle X-ray diffraction reveals muscle’s gearbox
Small angle X-ray diffraction reveals muscle’s gearbox
Skeletal muscles are the motors of the human body, they allow us to walk, run and breathe. Sometimes they need to lift heavy weights and at other times to make very fast movements against a lighter load. A man-made motor would typically use a gearbox to match its output to the load. Here, experiments show how muscles do the same.
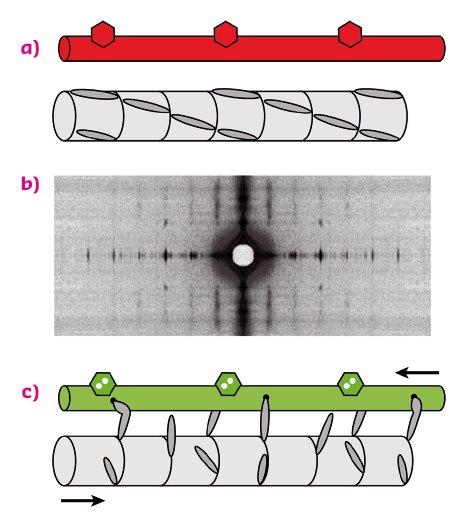
Contraction of skeletal muscles is driven by the relative sliding motion of two sets of filaments, thick filaments, mainly composed of the motor protein myosin, and thin filaments, containing the track protein actin (Figure 87a). Each myosin molecule has a long tail and two heads (only one is shown in the figure for simplicity), which are sometimes called motor domains because they can change shape when bound to actin (Figure 87c), driving the motion of the thin filaments. This shape change is coupled to the splitting of an ATP molecule, providing the energy for contraction. Contraction of an individual muscle cell is switched on by an electrical signal in its motor nerve, which triggers the release of calcium ions in the muscle cell; these calcium ions bind to thin filament regulatory proteins (hexagons in Figure 87a, c), causing a structural change in the thin filaments that allows the myosin motors to bind to them.
 |
|
Fig. 87: Arrangement of the thick and thin filaments in skeletal muscle and conventional model of thin-filament regulation. (a) resting muscle; (c) active contraction, triggered by calcium ions binding to thin filament regulatory proteins (hexagons). Red (green) denotes the OFF (ON) state of the thin filament. (b) X-ray diffraction pattern from a resting muscle fibre. |
In this textbook view of the regulation of muscle contraction, muscle is regulated by the track – the thin filament – rather than through the myosin motors themselves. This left a puzzle since the myosin motors in resting muscle lie in a helical array on the surface of the thick filament (Figure 87a), and it was not known how they sense the structure of the thin filament. Moreover, although it was clear that calcium ions are the trigger for contraction, it was not known how the response of the myosin motors is coupled to the load, i.e. how the gearbox works.
Experiments at beamline ID02 have now provided answers to these longstanding questions. X-ray diffraction patterns were recorded from single muscle cells (Figure 87b) while they were stimulated electrically under conditions whereby the length and load on the muscle cell could be controlled precisely. The very high brilliance and collimation of the X-ray beam combined with the high sensitivity and resolution of the 2D X-ray detectors overcame the major technical challenge of these experiments: to record X-ray signals from a single muscle cell on the millisecond timescale with very high spatial resolution.
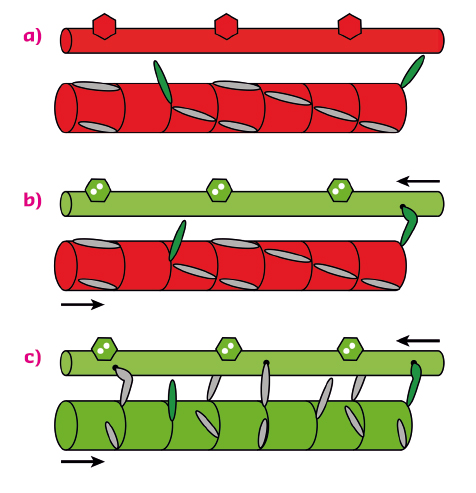
The results showed that, when the load on the muscle cell is kept low in the first few milliseconds after calcium activation, nearly all the myosin motors stay parked in the helical array on the surface of the thick filaments (Figure 88a, b). Only a few percent of the myosin motors are required to drive muscle shortening at high velocity after calcium ions have activated the actin filament. These constitutively active “switched ON” motors escape the helical packing (Figure 88a). However when the load is high, these motors generate a stress in the backbone of the thick filament which triggers a change in the packing of the myosin tails to one with a slightly longer periodicity along the filament axis (Figure 88c). This filament extension releases the remaining myosin motors from their helical parked state, making them available for actin binding and force generation against the high load. This intrinsic mechanical switch in the thick filament is the gearbox of muscle, enabling it to mobilise only a few myosin molecules when the external load is low at very low metabolic cost, and then switching on many more motors when they are needed to work against a high load.
 |
|
Fig. 88: The dual filament model of muscle regulation. (a) resting muscle; (b) and (c), calcium-activation at low and high load respectively. At high load (c), force generated by constitutively ON motors (green) switches the thick filament to a slightly longer axial periodicity, releasing the remaining motors. |
These results lead to a novel dual-filament concept of the regulation of contraction in skeletal muscle. In addition to the well-established structural change in the thin filaments controlled by calcium, a mechano-sensitive structural switch in the thick filament links muscle performance to its external load. This new paradigm of muscle regulation should help to guide therapeutic strategies to counter muscle weakness. Since the thick and thin filaments in heart muscle are very similar to those in skeletal muscle, dual-filament regulation may also control the heartbeat.
Principal publication and authors
Force generation by skeletal muscle is controlled by mechano-sensing in myosin filaments, M. Linari (a), E. Brunello (b), M. Reconditi (a), L. Fusi (b), M. Caremani (a), T. Narayanan (c), G. Piazzesi (a), V. Lombardi (a), M. Irving (b), Nature 528, 276-279 (2015); doi: 10.1038/nature15727.
(a) Università di Firenze, Firenze (Italy)
(b) King’s College London, London (UK)
(c) ESRF



