- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Matter at extremes
- Synchrotron X-ray spectroscopy sheds light on the major Cu-species in the Cu-CHA deNOx catalyst
Synchrotron X-ray spectroscopy sheds light on the major Cu-species in the Cu-CHA deNOx catalyst
The NH3-SCR reaction catalysed by Cu-exchanged zeolites is a promising way to remove hazardous NOx from diesel engine exhaust gases. Synchrotron-based spectroscopy unravelled the molecular-scale structure of Cu-sites in the dehydrated and working catalyst, a key step towards improved aftertreatment systems able to cut down harmful emissions from road traffic.
Road transport is a primary need in our everyday life and a major driving force of our economy. Unfortunately, the huge vehicle fleet moving people and freights all over Europe every day is also one of the major sources of air pollution in urban areas, with detrimental effects on human health and global ecology. The principal pollutants present in the exhausts from the increasingly popular diesel engines include mono-nitrogen oxides (NOx), particulate matter (PM), hydrocarbons (HC) and carbon monoxide (CO). The development of aftertreatment systems able to abate these noxious emissions, meeting the increasingly demanding air-quality standards, is an ongoing challenge, which nowadays attracts an enormous research interest.
 |
|
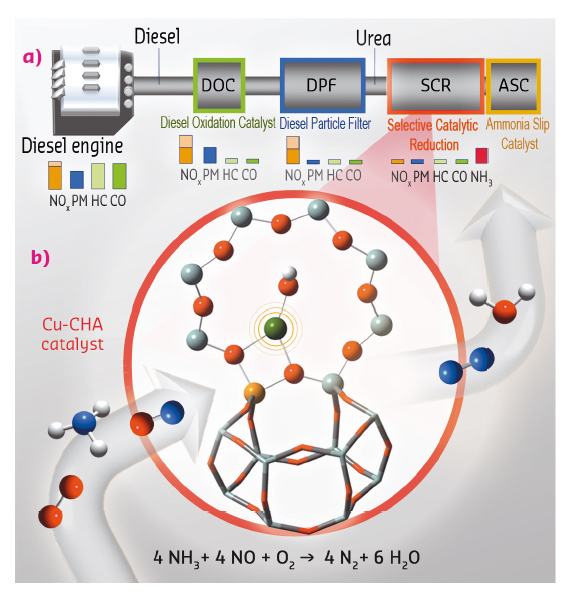
Fig. 111: (a) Schema of a state-of-the-art diesel aftertreatment system, showing the typical levels of major pollutants after each gas-cleaning unit. (b) Molecular-scale magnification of the deNOx unit, representing the NH3-SCR reaction on a Cu-CHA catalyst. |
The state-of-the-art options combine separate catalytic reactors for the different exhaust gas cleaning operations (Figure 111a). Here, molecular-level knowledge of the complex active phases in each cleaning unit is becoming an urgency to further improve the system performance. The most efficient deNOx units employ selective catalytic reduction (SCR), with the addition of excess ammonia, e.g. from urea. Among the various catalysts developed for NH3-SCR, the Cu-exchanged chabazite zeolite (Cu-CHA) has been selected as the best candidate for commercial applications due to its enhanced catalytic activity and hydrothermal stability (Figure 112b) [1,2].
 |
|
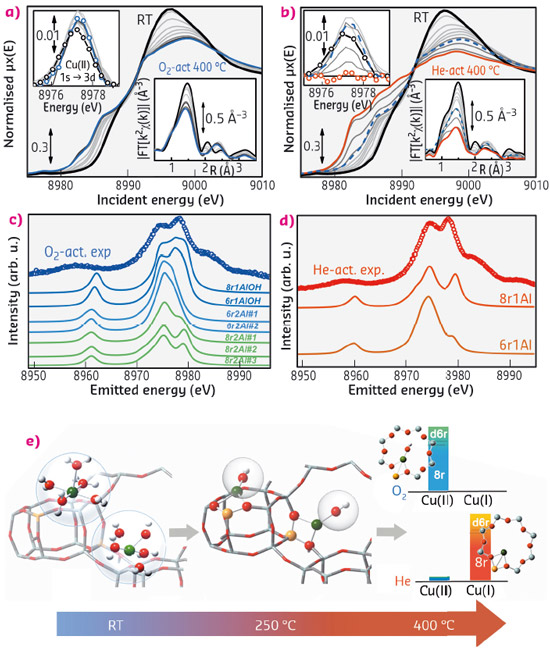
Fig. 112: (a,b) In situ XANES and EXAFS (insets) spectra collected at BM23 while heating the Cu-CHA catalyst from RT to 400°C in O2/He (a) and He flux (b); (c,d) Experimental XES spectra (coloured circles) measured at ID26 after dehydration in O2/He (c) and He flux (d) and corresponding theoretical curves from several DFT-optimised Cu(II) and Cu(I) sites in Cu-CHA; (e) Cu-speciation in the Cu-CHA catalyst as a function of the dehydration temperature and gaseous atmosphere, as derived from XAS and XES analysis. |
The combination of XAS and XES experiments at beamlines BM23 and ID26, respectively, allowed investigation of the Cu-species hosted in the CHA framework during the dehydration process as a function of temperature and gaseous environment, a crucial step towards unravelling the structure-properties relationships of this promising catalyst. A closed reactor cell connected to a gas rig allowed us to monitor the evolution of the copper coordination environment and oxidation state at realistic exhaust gas compositions and temperatures (Figure 112a, b). The major species present in the catalyst after dehydration were identified by DFT-assisted EXAFS fits and XANES/XES simulations (Figure 112c, d), considering several possible Cu(II) and Cu(I) sites in the CHA framework.
Data analysis revealed that the dehydration process of Cu cations is essentially completed at 250°C, with the formation of dehydrated [CuOH]+ species hosted in close proximity to 1 Al sites mostly in 8r units of the CHA zeolite. These species persist at higher temperature only if a certain amount of O2 is present in the gas feed, while in He-flux they undergo virtually total “self-reduction” as a consequence of an OH extra-ligand loss, resulting in bi-coordinated bare Cu+ cations (Figure 112e).
These results pave the way to the development of a consistent scheme for the SCR reaction on isolated Cu sites in Cu-CHA, able to produce the correct stoichiometry while allowing adsorption and desorption of only stable molecules [3]. XAS and XES studies at the ESRF, carried out in operando and in situ conditions at the BM23 and ID26 beamlines [3, 4], were determinant to experimentally validate the proposed reaction mechanism.
All these findings resulted in a greatly improved understanding of the SCR chemistry on Cu-CHA, and demonstrated the power of synchrotron-based spectroscopy in tackling the ‘catalyst characterisation challenge’ to design improved aftertreatment systems for cleaner road vehicles.
Principal publication and authors
Revisiting the nature of Cu sites in the activated Cu-SSZ-13 catalyst for SCR reaction, E. Borfecchia (a), K.A. Lomachenko (a,c), F. Giordanino (a), H. Falsig (b), P. Beato (b), A.V. Soldatov (c), S. Bordiga (a) and C. Lamberti (a,c), Chem. Sci. 6, 548-563 (2015); doi: 10.1039/C4SC02907K.
(a) Department of Chemistry, NIS Centre and INSTM Reference Center, University of Turin (Italy)
(b) Haldor Topsøe A/S, Lyngby (Denmark)
(c) Southern Federal University, Rostov-on-Don (Russia)
References
[1] U. Deka et al., ACS Catal. 3, 413-427 (2013).
[2] A.M. Beale et al., Chem. Soc. Rev. 44, 7371-7405 (2015).
[3] T.V.W. Janssens et al., ACS Catal. 5, 2832-2845 (2015).
[4] F. Giordanino et al., J. Phys. Chem. Lett. 5, 1552-1559 (2014).



