- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Structure of materials
- Formation and evolution of a high catalytic performance carbon supported Pd sulfide nano-phase
Formation and evolution of a high catalytic performance carbon supported Pd sulfide nano-phase
The combination of high-energy X-ray diffraction (HEXRD) and mass spectroscopy (MS) measurements reveals that the reduction under hydrogen of the carbon supported palladium sulphate precursor provides a Pd4S based catalyst with significant activity and superior selectivity to partial hydrogenation of dialkenes, and high stability under reaction conditions.
Partial hydrogenation of alkynes and alkadienes in the presence of alkenes is one of the most important processes in the chemical industry and its selectivity is a relevant issue. Supported palladium catalysts are currently used for the hydrogenation of 1,3-butadiene (Bd), but because they lack selectivity for the desired n-butenes when the ratio of monoalkenes to dienes is high, promoters (Ag, Au, Ga or Cu) or modifiers (CO or sulfur) are needed to enhance the yield of partially hydrogenated products [1]. These alternatives suppose complex preparation methods or continuous feeding of a modifier compound to maintain the catalyst properties during reaction [1, 2].
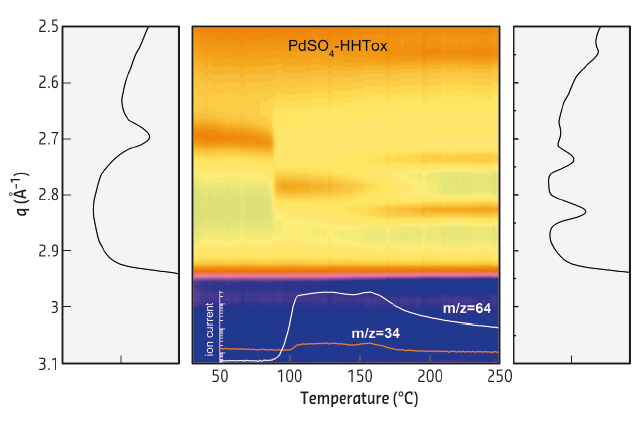
We present here a new procedure for the preparation of palladium sulfide nanoparticles, which are deposited-anchored over highly graphitised carbon nanofibres. The preparation method is based on the use of PdSO4 as metal precursor or alternatively in the previous functionalisation of the carbon surfaces with sulfonic groups by treatment with fuming sulfuric acid. Using “in situ” high-energy X-ray diffraction coupled with mass spectroscopy at the ID15A beamline, for both cases it was demonstrated that the initially present palladium hydride is transformed into a palladium sulfide (Pd4S) during the reduction treatment. Figure 46 shows the structural evolution of the sample prepared with palladium sulfate as measured with HEXRD. When the sample is exposed to hydrogen/helium at room temperature the HERXD pattern reveals the formation of the β phase of Pd hydride (peak (111) at 2.70 Å−1) which is transformed into metallic Pd at 90°C (2.80 Å−1). As the temperature approaches 150°C, a second transformation occurs, resulting in the formation of the Pd4S phase ((210) and (112) peaks at 2.74 and 2.83 Å−1, respectively). The formation of the Pd sulfide phase is further described with the help of MS. From the MS analysis (inset, lower panel), we observe the evolution of H2S (m/z = 34) and SO2 (m/z = 64). Both products stem as main gas residues coming from the decomposition/reduction of the sulfate anions of the metal precursor deposited on the support and appear with a constant level from 100 to 160°C. At this latter temperature, Pd is completely transformed to the single well crystallised Pd4S structure.
 |
|
Fig. 46: Intensity contour maps of the HEXRD patterns obtained during temperature-programmed reduction in hydrogen/helium from room temperature up to 250°C. |
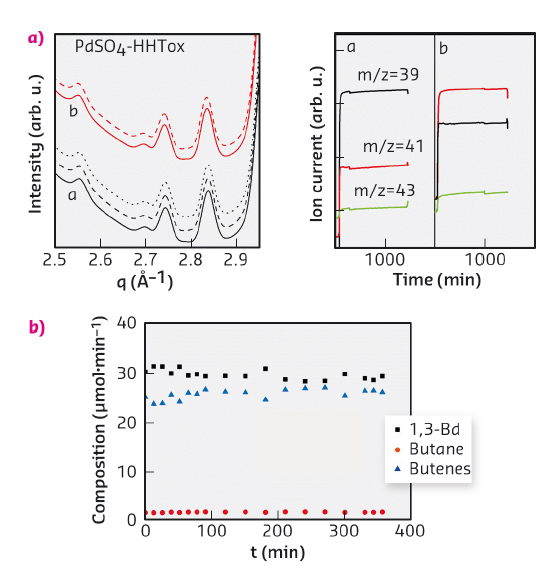
Subsequently, we studied the evolution of Pd phases under butadiene hydrogenation reaction atmosphere at different temperatures and during H2/(H2+Bd)/H2 alternate exposure. Once the catalysts were treated in hydrogen at 250°C, the temperature was reduced to the corresponding reaction temperatures. Figure 47 shows an HEXRD study carried out on the sample prepared with palladium sulfate. No structural changes were noticed in the phase formed during reduction in hydrogen at 250°C during the time of exposition to reaction mixture and switching to hydrogen at both 100 and 150°C temperatures. The MS analysis shows the high selectivity of the catalyst for the partial hydrogenation of butadiene (m/z = 39) into butenes (m/z = 41). The stability of the Pd4S phase under reaction conditions was also proved by a long-term catalytic run performed in a fixed bed reactor at 100°C. Activity and selectivity were maintained throughout the run.
 |
|
Fig. 47: (a) HEXRD patterns obtained under consecutive H2 (full line), H2 + Bd (dashed line), H2 (dotted line) atmospheres at several constant temperatures (a: 100°C; b: 150°C) and MS results for selected m/z values under H2 + Bd. (b) Stability test carried out at 100°C. |
These XRD-based results suggest that reduction under hydrogen of the carbon supported palladium sulfate precursor should provide a facile method to synthesise a well-defined single palladium sulfide structure which has significant activity, appropriate selectivity, and high stability under reaction conditions.
Principal publication and authors
Detecting the genesis of a high-performance carbon-supported Pd sulfide nanophase and its evolution in the hydrogenation of butadiene, B. Bachiller-Baeza (a), A. Iglesias-Juez (a), E. Castillejos-López (b), A. Guerrero-Ruiz (b), M. Di Michiel (c), M. Fernández-García (a) and I. Rodríguez-Ramos (a), ACS Catal. 5, 5235−5241 (2015); doi: 10.1021/acscatal.5b00896.
(a) Instituto de Catálisis y Petroleoquímica, CSIC, Madrid (Spain)
(b) Departamento de Química Inorgánica y Química Técnica, UNED, Madrid (Spain)
(c) ESRF
References
[1] A. Cooper et al., Catal. Sci. Technol. 4, 1446−1455 (2014).
[2] B. Bachiller-Baeza et al., Catal. Today 249, 63−71 (2015).



