- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Structure of materials
- Polymorphism on the nanoscale: unravelling structural diversity in Au144s(SR)60 nanoclusters
Polymorphism on the nanoscale: unravelling structural diversity in Au144s(SR)60 nanoclusters
In the bulk, gold has a simple structure with atoms arrayed in a regular fashion, while on the nanoscale it can have exotic structures, with new properties and functionalities. This work used intense X-ray beams on tiny samples of nanosized gold clusters that revealed polymorphism: gold clusters can have doppelgängers.
The advancement of material science builds on understanding the intricate relations between atomic structure and properties of a material. For the last 100 years, Bragg diffraction has been the tool of choice to map this relation by providing us with the possibility to determine atomic arrangements in solids with high precision. However, the conventional crystallographic techniques fail when it comes to nanomaterials, and this ‘nanostructure problem’ represents one of the big challenges in nanotechnology [1]. Now, with the high-energy, high-flux X-rays available from third-generation synchrotrons, we are getting closer to a solution, as new possibilities emerge for structural characterisation. Here, we have used high energy X-ray total scattering at beamline ID11 to solve the structure of Au144(SR)60, and show that these small gold clusters exist in two different structures; illustrating polymorphism on the nanoscale.
‘Magic sized’ metal nanoclusters, such as Au144(SR)60, represent a new material class between particles and molecules: unlike nanoparticles, they are atomically monodisperse meaning that the exact number of metal atoms and stabilising ligands can be controlled. While the composition of the clusters can be determined from mass spectrometry, the arrangements of the atoms remain undetermined. Atomic structure with only short-range order gives rise to diffuse scattering of X-rays, which cannot be analysed with conventional crystallographic techniques. Instead, structural information from diffuse scattering data can be extracted by collecting data to high scattering angles, and subsequently Fourier transforming the data to obtain the pair distribution function (PDF). The PDF represents a histogram of interatomic distances in the sample, allowing structural information to be extracted [2]. In this study, we used PDF to determine the atomic arrangement in the Au144(SR)60 cluster, where ‘SR’ represents a thiol ligand.
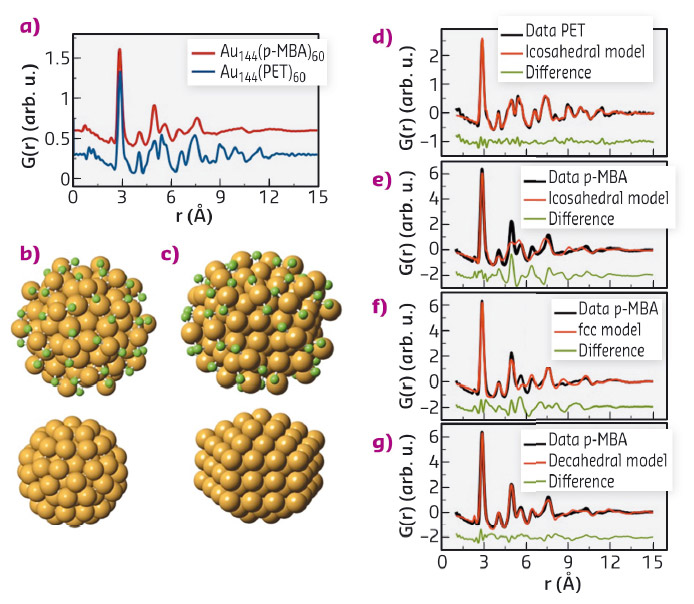
Figure 54a shows PDFs obtained from Au144(SR)60 clusters covered with two different thiol ligands; phenylethane thiol (PET) and para-mercaptobenzoic acid (pMBA). Surprisingly, we see distinct differences between the two PDFs, despite both samples containing clusters of exactly 144 gold atoms and 60 thiol ligand molecules. The data were modelled using our new software for PDF analysis, diffpy-CMI [3]. We initially tested a structure model deduced from DFT calculations [4]. This structure has a 114 atom icosahedral core, while the surface is covered by 30 –S-Au-S- ‘staple’ units, as illustrated in Figure 54b. As can be seen from Figure 54d-e, this structure fits well with the PET data, but does not describe the pMBA covered clusters which thus have a fundamentally different structure: the clusters exhibit polymorphism.
 |
|
Fig. 54: a) PDFs obtatained for two different Au144(SR)60 clusters. b) Full icosahedral model (top) and 114-atom core (bottom). c) Full decahedral model (top) and 114 atom core (bottom). Gold atoms are shown in orange, while green represent sulfur. d-g) PDF fits. |
To determine the structure of the pMBA clusters, we first attempted fitting the bulk gold fcc structure to the data, as shown in Figure 54f. While not being a physical model for the cluster, the simple fcc structure model fits the data better than the icosahedral model. This result lead us to explore a series of fcc-derived models, including clusters in the Marks decahedron shape; built up from 5 small twinned fcc structures illustrated in Figure 54b. A decahedral 114 atom core, covered by 30 staples gave an excellent fit (Figure 54g) fully describing the data in a physical manner.
Two distinct structures thus exist for the Au144 clusters, one with an icosahedral core (form I), and a second polymorph with a decahedral core (form II). Surprisingly, we found that for certain thiol ligands, both polymorphs were present in the same sample. This result illustrates that the structural diversity is not a simple effect of e.g. ligand identity, but indicates that the two structures are very close in energy. The discovery of polymorphism adds a new dimension to nanoengineering, beyond control of particle size and morphology. The two structures seen in our data furthermore suggests many new studies of gold nanoclusters. Polymorphism may not be limited to the Au144(SR)60 cluster, but could exist in many different materials systems. Recently, several new metal clusters have been isolated in the size range from 50 to 300 atoms. The structures of many of these clusters remain undetermined. We believe that PDF is an excellent tool for these studies and will aid in providing full structure solutions to many new nanomaterials.
Principal publication and authors
Polymorphism in magic-sized Au144(SR)60 clusters, K.M.Ø. Jensen (a), P. Juhas (b), M.A. Tofanelli (c), C.L. Heinecke (c), G. Vaughan (d), C.J. Ackerson (c) and S.J.L. Billinge (a,b), Nature Communications 7, 11859 (2016); doi: 10.1038/ncomms11859.
(a) Department of Applied Physics and Applied Mathematics, Columbia University, New York (USA)
(b) Condensed Matter Physics and Materials Science Department, Brookhaven National Laboratory, Upton (USA)
(c) Department of Chemistry, Colorado State University, Fort Collins, Colorado (USA)
(d) ESRF
References
[1] S. Billinge et al., Science 316, 561-565 (2007).
[2] S. Billinge et al., Chem. Commun. 749-760 (2004).
[3] P. Juhas et al., Acta Cryst. A71, 562-568 (2015).
[4] D. Bahena et al., J. Phys. Chem. Lett. 4, 975-981 (2013).



