- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Complex systems and biomedical sciences
- Virus-like capsids from the lattice assembly of non-peptide small molecules
Virus-like capsids from the lattice assembly of non-peptide small molecules
The lattice of cyclodextrin complexes self-assembles into a variety of capsid-like structures such as lamellae, helical tubes, and hollow rhombic dodecahedra. In particular, the resemblance to virus capsids goes beyond morphology and extends to structural rigidity and crystallinity – a well-defined, universal, 2D rhombic lattice of molecular arrangement.
All living organisms are self-assembled entities where two major kinds of self-assembly are involved. One is the assembly of lipids into soft, fluidic membranes mainly driven by the hydrophobic interaction. The lipid assembly is extensively reproduced and well extended by synthetic amphiphilic small molecules, polymers, and even nanoparticles to form lamellar, tubular, vesicular, and micellar structures (Figure 60a). The other kind is the assembly of proteins into rigid, crystalline structures driven by a combination of hydrophobic, H-bonding and electrostatic interactions. For example, virus capsids are rigid protein shells often in helical or icosahedral structures. The protein assembly that produces crystalline structures (Figure 60b) such as lamellae, tubules, and polyhedra is, however, largely unparalleled by synthetic or non-peptide molecules with a few notable exceptions. Looking beyond biomimetic self-assembly, one can notice that carbon allotropes share the same morphological pattern: graphite, graphene, nanotubes, and C60 in analogy to lamellar, tubular, and polyhedral assemblies. In this context, what has been gradually recognised is the importance of intermolecular interactions, structural flexibility/rigidity, and fluidity/crystallinity over that of morphology. A few building blocks were reported to successfully mimic protein assemblies’ morphologies but cannot rival their rigidity nor well-defined crystallinity.
 |
|
Fig. 60: Stylised view of the self-assembly of lipids, proteins, and cyclodextrin complexes. a) Lipid molecules form lamellar, tubular, and vesicular structures, the flexibility and fluidity of which are emphasised. b) Proteins form lamellar, helical tubular, and regular icosahedral structures with rigidity and hexagonal crystallinity. c) SDS@2β-CD assembles with inherent rigidity and in-plane, rhombic crystalline nature. |
Seeking a synthetic system that parallels the morphology, rigidity, and crystallinity of protein assemblies or virus capsids, it was decided to study a supramolecular complex, SDS@2β-CD (one sodium dodecyl sulfate molecule inside two β-cyclodextrin molecules, Figure 60c). This work reports the lattice self-assembly of SDS@2β-CD into a variety of capsid-like structures such as lamellae, helical tubes, and hollow rhombic dodecahedra. The dodecahedral morphology has not hitherto been observed in self-assembly systems. The tubes can spontaneously encapsulate colloidal particles and liposomes. The dodecahedra and tubes are respectively comparable to – and much larger than – the largest known virus. In particular, the resemblance to protein assemblies is not limited to morphology but extends to structural rigidity and crystallinity – a well-defined, 2D rhombic lattice of molecular arrangement is strikingly universal for all the observed structures.
 |
|
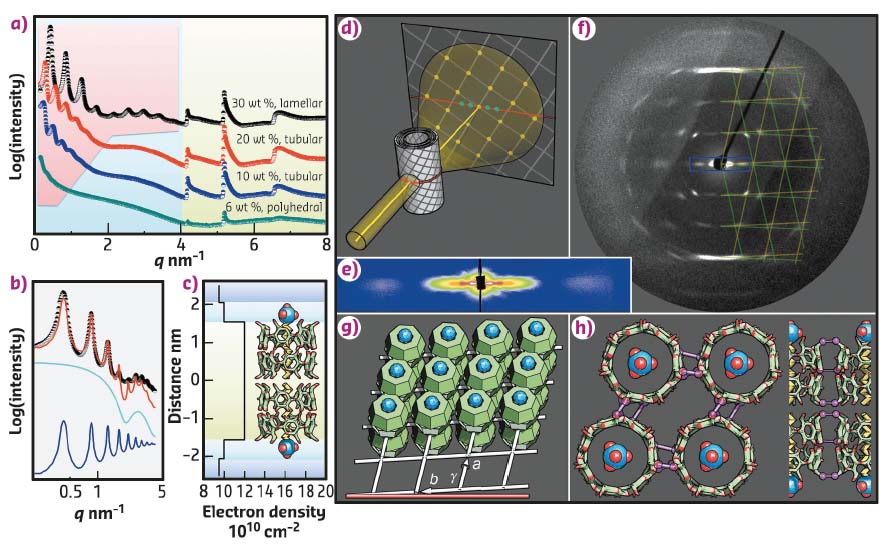
Fig. 61: X-ray scattering of the SDS@2β-CD structures. a) SAXS curves of the lamellar, tubular, and polyhedral phases with different SDS@2β-CD concentrations. b) The SAXS curve up to 4 nm-1 is fitted by a bilayer form factor (cyan line) and lamellar structure factor (blue line) with a good result (red line). c) The electron density profile across the bilayer. d) A scheme of WAXS of aligned multilamellar tubes with in-plane crystallinity. e) Scattering pattern along the equator at small angle, corresponding to the blue box in f. f) Scattering pattern at large angle is super-positioned by the yellow and green grids. g) The in-plane lattice. h) Top and side views of the SDS@2β-CD lattice, highlighting the possible, inter-CD H-bonds (magenta). |
Critical to determining the capsid-like structures and molecular arrangements are the X-ray results obtained on the DUBBLE SAXS/WAXS beamline BM26B. The SAXS curves (Figure 61a) feature equally spaced, lamellar peaks that gradually vanish upon the dilution of SDS@2β-CD, indicating the increase of layer-to-layer distance and the lessening of layers in a stack. The scattering curves up to 4 nm-1 were fitted by a bilayer form factor and lamellar structure factor (Figure 61b). The corresponding electron density profile suggests a bilayer arrangement of SDS@2β-CD with its long axis perpendicular to the layer (Figure 61c). The scenario of a multilamellar tube diffracting X-rays is schematically illustrated in Figure 61d. In line with this scenario, a few lamellar peaks and a broad form factor peak are located on the equator at small q (Figure 61e), while a well-defined diffraction pattern is observed at large q (Figure 61f). The pattern’s symmetry along the equator and medial axis signifies a helical nature for the tubes. The pattern is perfectly matched by two mirrored lattices (green and yellow grids). The in-plane unit cell is thus resolved as a rhombus with a = b = 1.52 nm (comparable to β-CD diameter, 1.5 nm) and γ = 104°.
Principal publication and authors
Giant capsids from lattice self-assembly of cyclodextrin complexes, S. Yang (a), Y. Yun (b), J. Huang (b), A.V. Petukhov (c, d), L.M.J. Kroon-Batenburg (c), M. Drechsler (e), C. Zhou (b), M. Tu (a), S. Granick (f) and L. Jiang (a), Nat. Commun 8, 15856 (2017); doi:10.1038/ncomms15856.
(a) Jinan University, Guangzhou (China)
(b) Peking University, Beijing (China)
(c) Utrecht University, Utrecht (The Netherlands)
(d) Eindhoven University of Technology, Eindhoven (The Netherlands)
(e) University of Bayreuth, Bayreuth (Germany)
(f) Institute for Basic Science, Ulsan (Republic of Korea)



