- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Electronic structure, magnetism and dynamics
- Understanding spin-orbit entanglement in molecular osmates
Understanding spin-orbit entanglement in molecular osmates
Molecular model systems may help to unravel the interplay between chemical environment and physical properties in 5d transition element-based materials. Such elements feature strong coupling of the spin of outer electrons to their orbital motion around the nucleus, which can affect physical properties, depending on atomic-level environments and metal oxidation state.
The spin-orbit interaction leads to a plethora of exotic physical properties in condensed matter. It is particularly true for 5d metal ion-based materials since this interaction gets dramatically stronger with increasing atomic number. The combined action of the crystal field and the spin-orbit interaction results in an entanglement of the spin and orbital degrees of freedom. Exotic physical properties associated to this entanglement have been recently reported in, for example, osmium-based materials [1,2].
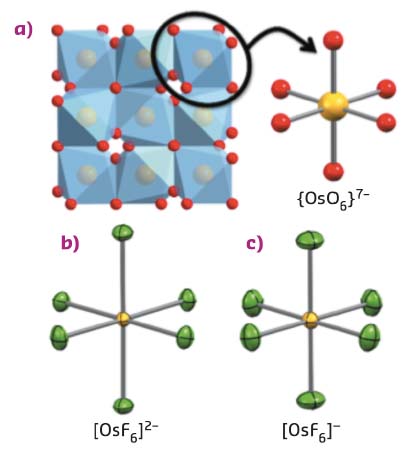
Tailoring the electronic properties of such materials would require a fundamental understanding of the spin-orbit interaction at the single-ion level. Molecular chemistry provides the methodology to spatially isolate metal ion modules and can thereby minimise or supress long-range interactions between magnetic sites present in solids, e.g. oxides (Figure 111a). However, finding molecular entities, which can realistically emulate metal ion sites in solids, is a complicated task. In nearly all oxides of the 5d elements, the metal ions are surrounded by six oxide ions (O2–) placed at the corners of an octahedron. The closest, chemically attainable, molecular model of this structural motif is [OsF6]x–, which exists with two oxidation states of the osmium ion; [OsIVF6]2– and [OsVF6]–. The possibility to switch the oxidation state between OsIV and OsV provided a great opportunity to elucidate the spin-orbit physics in osmates.
 |
|
Fig. 111: Schematic illustration showing the conceptual link between osmium oxides, here represented by a) the structure of Cd2Os2O7, b) the molecular fluoride-based analogues [OsIVF6]2– and c) [OsVF6]– . Colour code: Os, yellow; O, red; F, green. Cd is omitted for clarity. |
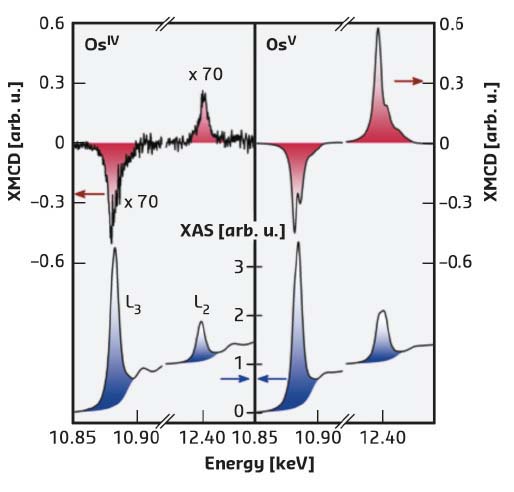
X-ray absorption (XAS) and X-ray magnetic circular dichroism (XMCD) were carried out at ID12 and spectra at the L2,3 edges of osmium in both [OsIVF6]2– and [OsVF6]– are shown in Figure 112. A clear XMCD signal is observed in both cases, although it is nearly two orders of magnitude larger for OsV than for OsIV, due to a much larger magnetic moment of the former. The combined analysis of the XAS and XMCD data offers a unique possibility to disentangle the spin and orbital contributions to the total magnetic moment. In the reported molecular osmates, the experimental results (Figure 112) demonstrate the van Vleck paramagnetic behaviour of the OsIV centre with an orbital magnetic moment contributing to 25% of the total magnetisation. In the Curie-type paramagnetic OsV system, the orbital contribution to the magnetic moment is expected to vanish due to the orbitally non-degenerate ground state. However, a finite orbital moment that did not exceed 5% of the total magnetisation was found. To further elucidate the physical origin of these magnetic moments, ab initio ligand field theory calculations (AILFT) were performed for the first time on a 5d metal ion complex. The calculations reproduce the experimental results and demonstrate that the orbital moment in OsV originates from admixture of the electronic ground state with energetically high-lying states. Indeed this effect becomes possible only because of the interplay of the strong spin-orbit interaction with the ligand field.
 |
|
Fig. 112: X-ray absorption (blue) and XMCD (red) spectra of [OsIVF6]2– (left) and [OsVF6]– (right) obtained at ID12 at T = 3 K under a magnetic field of H = 17 T. |
Surprisingly, X-ray spectroscopic data on osmium-based materials are still scarce in literature, which is rather intriguing given the broad interest in spin-orbit entangled phenomena. This work demonstrates that the molecular chemistry approach to elucidating the relationships between oxidation state, local structure and spin-orbit entanglement is certainly a viable approach for understanding the exotic physical properties of novel 5d-based materials.
Principal publication and authors
[OsF6]x–: Molecular Models for Spin-Orbit Entangled Phenomena, K.S. Pedersen (a,b,c,d,j), D.N. Woodruff (e), S.K. Singh (f), A. Tressaud (c,d), E. Durand (c,d), M. Atanasov (f,g), P. Perlepe (a,b,c,d), K. Ollefs (h,k), F. Wilhelm, (h), C. Mathonière (c,d), F. Neese (f), A. Rogalev (h), J. Bendix (i) and R. Clérac (a,b). Chem. Eur. J. 23, 11244-11248 (2017); doi: 10.1002/chem.201702894.
(a) CNRS, CRPP, UMR 5031, Pessac (France)
(b) Univ. Bordeaux, CRPP, UMR 5031, Pessac (France)
(c) CNRS, ICMCB, UMR 5026, Pessac (France)
(d) Univ. Bordeaux, ICMCB, UMR 5026, Pessac (France)
(e) Department of Chemistry, University of Oxford (UK)
(f) Max-Planck Institut für Chemische Energiekonversion, Mülheim an der Ruhr (Germany)
(g) Institute of General and Inorganic Chemistry, Bulgarian Academy of Sciences, Sofia (Bulgaria)
(h) ESRF
(i) Department of Chemistry, University of Copenhagen (Denmark)
(j) DTU Chemistry, Technical University of Denmark, Kgs. Lyngby (Denmark)
(k) Faculty of Physics and Center for Nanointegration Duisburg-Essen (CENIDE), Universität Duisburg-Essen, Duisburg (Germany)
References
[1] S. Calder et al., Nat. Commun. 7, 11651 (2016)
[2] L. Lu et al., Nat. Commun. 8, 14407 (2017)



