- Home
- Users & Science
- User Guide
- Before your experiment
- Remote experiments on non Structural Biology beamlines
Remote experiments on non Structural Biology beamlines
Since 2020, remote experiments can be carried out at the ESRF, without the physical presence of the users on site. In a remote experiment, samples are made available to the concerned ESRF beamline staff and the experiment is carried out via remote communication between the users and the local contact.
In these pages, you will find instructions for users to allow you to correctly prepare, organize and run your remote experiment.
The workflow for remote experiments is significantly different from what you are used to for onsite experiments. Due to the need to send samples to the ESRF, sample sheets are required and the declaration of users and samples in the A-form is different. There is also a specific remote safety training to follow and an experiment protocol document to complete together with your Local Contact. Finally all samples and/or equipment must be sent using our ICAT sample Tracking system and via a recognized courier company (FedEx, UPS, DHL, …). Instructions for all of these points can be found in these pages.
Instructions for your Remote Experiment
Before the Experiment
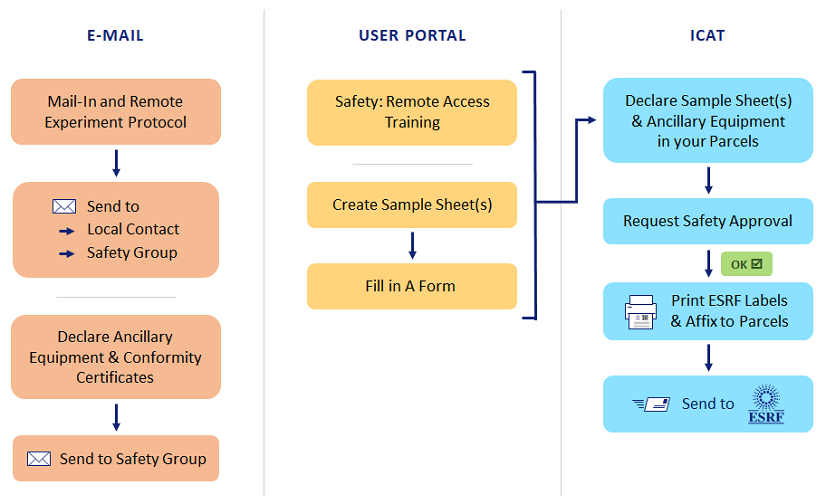
- All samples, references and chemical products for your proposal must be declared via the submission of a “Sample Sheet” for each different type of sample, reference of chemical product. Multiple samples of the same type are declared in a single sample sheet (e.g. for 20 crystals of the same material, only one sample sheet is required).
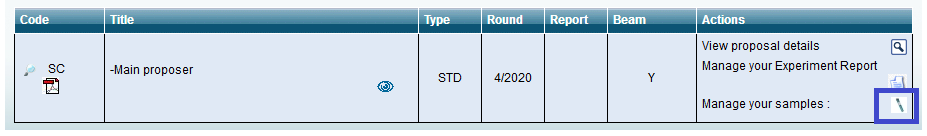
- Sample sheets are created via the User Portal by going to the list of your finalized proposals and clicking on the icon next to “Manage your samples” for the proposal in question.
- Your sample sheet(s), once submitted, will be evaluated by the ESRF Safety Group and assigned a colour (green, yellow or red). Once a sample sheet has been assigned a colour by the Safety Group, it can be added to the A-Form.
Declaring the users – tab “Users”:

- All users who are participating in the experiment should be declared, even when the experiment is taking place remotely.
- For each user declared, please tick on the “remote user” tickbox – this will deactivate the Guesthouse, Travel Office and Reimbursed tickboxes (please see the relevant section on reimbursement of shipping costs later in these web pages). The arrival and departure dates must still be filled in, corresponding to the dates when the user will be involved in the remote experiment (start and end dates of the experiment, in most cases).

Declaring the samples (including references and any chemical products) – tab “Samples”:

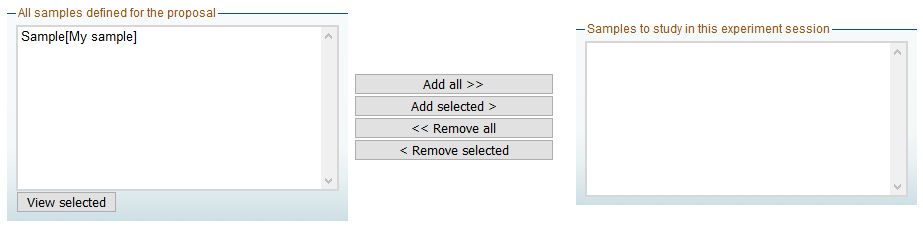
- In the Samples tab of the A-Form, you will see the list of all sample sheets that you have declared for the proposal in question, and that have been approved and assigned a colour by the Safety Group.
- Click on the samples sheets that are relevant for the particular experiment session that you are preparing and click on “Add selected”, or on “Add all” if all samples are to be measured during this experiment session.
Declaring your sample environment – tab “Sample Environment”:

- Fill out the obligatory fields of this tab. Please note that in the ‘Sample Environment’ sub-section, certain environments are indicated as not available for Mail-In experiments – this means that these environments cannot be sent but in most cases the equivalent ESRF equipment can be used on site. Details are given in the “Mail-In and Remote” Safety Training.

- For all non-Structural Biology mail-in and remote experiments, the user team must write down clearly what is expected of the Local Contact and what aspects of the experiment the user team will take care of, so that all parties know what is expected before the experiment starts.
- This is done by completing the “Mail-In and Remote Experiment Protocol” document which is attached to the Invitation Letter email, sent by the ESRF to the Main Proposer.
- A representative of the user team must write the protocol and then send the document by email to both the Local Contact and to the ESRF Safety Group (expsaf@esrf.fr). The Local Contact will then accept or annotate the protocol by filling in his/her own comments in the appropriate section, and send back to the user team and Safety Group.
- Once the user team and Local Contact have converged and agreed on the protocol, both parties will tick the appropriate tickbox and the user team will send the final document to the Local Contact and ESRF Safety Group.
- As always, all users declared on an A-Form must complete the appropriate Safety training (valid one year) and validate the User & Safety Declaration form, otherwise they will not be recognized as a user on that particular experiment.
- A specific safety training module exists for users participating remotely in an ESRF experiment. All users added to an A-Form and indicated as “remote user” MUST complete the Remote Access training.
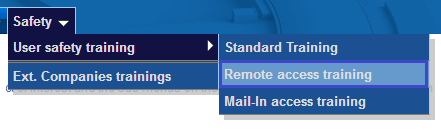
- In particular this training explains what users can and cannot send to the ESRF so please read the information carefully. Any unauthorized substance that is sent to the ESRF will be destroyed.
ICAT Sample Tracking
All samples, references, chemical products and equipment related to user experiments (except on the Structural Biology beamlines) must be sent to the ESRF using our ICAT Sample Tracking software, and then via a registered courier company such as FedEx, UPS, DHL, etc. Any shipment of samples by normal postal services is FORBIDDEN. For detailed instructions on using the ICAT Sample Tracking application, please click here.
- Any proposer or user on a proposal can create a shipment and link it to the appropriate experiment session and A-Form.
- Within that shipment, users can create one or several parcels to send to the ESRF, and in each parcel the users can declare both sample sheets and ancillary equipment.
Please keep in mind that the ESRF will pay shipping costs for up to 3 parcels (4 for LTPs and 2 for Cryo-EM experiments) for public beam time experiments only - see below.
- Once a parcel is ready to be sent, it has to be approved for sending by the ESRF Safety Group. Once it has been approved, a parcel label becomes available and must be printed and affixed to the parcel by the users.
- This label contains the address and a QR code which allows the parcel to be tracked.
- Any parcels containing samples, substances or equipment sent for a user experiment and that do not display this label will be destroyed, since they will not have been approved for sending to, and reception by, the ESRF by the Safety Group.
IMPORTANT : Make sure to request a return label from your courier company for it's seamless return to you after your experiment. If you will use FedEx, for example, select "Include a return label" in the shipping details.
Transport administration
Users and their institutes are responsible for sending their parcels and for declaring them correctly to the courier company. Please consult with your home institution safety transport advisor for instructions and regulations on shipping procedures and Incoterms® applying to your case. Be aware that a proforma invoice (for outside CEE countries) or customs invoice is always to be issued in the name of the user and only care of (c/o) ESRF.
Transport insurance: When declaring your shipment with your courier company, please put a transport insurance value of 0€. It is possible that your home institution has an insurance covering the transport of goods and that you don’t have to use the courier company insurance. Otherwise, if you wish to put a non-zero value, this is at the discretion of the user, and resulting courier company transport insurance costs for the shipment will not be reimbursed by the ESRF.
Customs value: Please put 10€ for parcels containing samples. For parcels containing equipment, we recommend that you give the real value of the equipment. This is in case you wish to make any claim to your insurance company for damage or loss, to demonstrate that the equipment has real monetary value. For equipment sent from non-EU countries, the customs value declaration may result in the need for the ESRF to open a temporary import dossier, which may cause a delay of a few days in the delivery of your parcel. Please take this into account and send your parcel in plenty of time for your experiment.
Particular care must be taken when the parcel has content that is considered as “dangerous goods”, and in general we advise to list the content of the parcels with eventual material safety data sheets (MSDS).
Transport of Dangerous Goods
In the case of transport of dangerous goods, the user team and their institutes must ensure that they comply with all necessary procedures and labelling, according to the advice of their safety transport advisor and the courier company used.
When seeking Safety approval for sending a parcel in the ICAT sample tracking software, the users will be asked to indicate whether the parcel contains dangerous goods, and must be careful to reply correctly so that the ESRF is aware when parcels contains dangerous goods and can apply the appropriate procedures on site and for return of the parcel.
When to send the samples
It is the responsibility of the users and their institutes to send their shipment on time for their experiment. It is important to leave at least 3 days between sending the parcels and the experiment start. If a problem occurs on a 24 hour delivery, it will NOT arrive on time and the beam time will be wasted. For equipment/goods dispatched from outside the EU, the shipping should include a larger time buffer. Ensure that the delivery takes place during week days 08:30-12:00 and 13:15-17.00. The ESRF stores are closed at the weekends and for French and ESRF holidays and no deliveries can take place on any of these days. The ESRF stores can be reached at +33 (0)4 76 88 2733 during their working hours.
Due to COVID-19 restrictions, all parcels sent to the ESRF are kept in quarantine for 24 hours. We advise you to send your samples well in advance to take this quarantine in account before your experiment starts.
During the Experiment
Section under construction.
Section under construction.
Section under construction.
Section under construction.
After the Experiment
- The ESRF will reimburse the cost of shipping a parcel in place of paying for a user trip. For most experiments, this means the ESRF will pay the shipping costs for up to 3 parcels (4 for LTPs and 2 for Cryo-EM experiments).
Important note : the ESRF will not reimburse any additional costs related to transport insurance or customs clearance for the shipment of the parcels; these costs must be covered by the user or the user institute (see the section Before Your Experiment - Sending your Samples - Sample Transport further up in this page).
- To request reimbursement, one of the members of the user team must submit a reimbursement claim (Excel file downloadable here), indicating clearly the number of parcels for which reimbursement is requested, the individual cost for each parcel, and the total cost for the shipment of the parcels. Please send your reimbursement claim to useroff(at)esrf.fr. Detailed information on reimbursement claims can be found here (section 8. Reimbursement procedure).
- As for the reimbursement of user travel, only parcels that are sent to/from ESRF member countries will be reimbursed. The parcels do not necessarily have to be sent from the laboratories of the users on the A-form.
- The ESRF cannot reimburse an institute, only a user. Therefore, the ESRF will reimburse the user who has submitted the reimbursement claim. All reimbursed users should arrange directly with their institute for reimbursement of costs if they have been paid by the institute.
- Don’t forget to submit your experiment report after your experiment has finished, whether it be onsite or remote. The deadline for submitting your report is 3 months after the experiment, or the next proposal submission deadline if it should support a new request for beam time.
Liability and Responsibility
ESRF will take the necessary precautions for the proper reception and use of samples and auxiliary equipment supplied by the user in the frame of a remote experiment (mail-in or remote access experiment), or sent ahead of an onsite experiment.
The above notwithstanding, the ESRF and the user, having agreed that the experiment will take place remotely, acknowledge that in no event shall the ESRF be held liable for the loss of samples and/or auxiliary equipment provided by the user, nor for any damage which may be caused to them. This applies during their transport to/from the ESRF, their storage, their handling and, more generally, during all operations in relation to the experiment.
The user thus undertakes not to claim damages from the ESRF for the loss or damage of samples and/or equipment provided for a remote experiment or sent ahead of an onsite experiment, except in the case of wilful misconduct or gross negligence by the ESRF.
The user team and their institutes are responsible for all import(s) into and/or export(s) from France relevant to the user experiment. The same applies to re-import and re-export of materials. The user team and their institutes must ensure that all import and export regulations of all countries concerned are complied with, for example customs law, tax law, foreign trade law, regulations concerning transport of dangerous goods.



