- Home
- News
- General News
- Manufacturing defects...
Manufacturing defects in silicon-based Li-ion batteries trigger degradation
15-05-2024
Scientists have found that the important origin of ageing of silicon-based Li-ion batteries is in the electrode processing during the manufacturing. They combined X-ray and neutron imaging techniques at the ESRF and the Institut Laue Langevin (ILL) to observe the microstructural changes during charging and discharging. Their results are out this week in Energy and Environmental Science.
Share
Li-ion batteries are widely used in mobile devices, transportation and energy storage, but they have limitations, including durability and degradation over time. When and why defects and failure appear in commercial batteries is still mostly unknown.
Now scientists from the ESRF, the ILL and the CEA-IRIG, Materials Center Leoben Forschung GmbH and battery manufacturer VARTA Innovation GmbH have used non-destructive X-ray and neutron imaging at the ESRF and ILL, respectively, to determine one of the origins and causes of the degrading mechanism in silicon-based Li-ion batteries.
X-rays and neutrons analysis
The samples were industrially graded batteries that are currently being tested for future commercialisation and that include large amount of silicon. The difference with current batteries lies in the anode, which is often made of state-of-the-art graphite in commercial batteries. In the new batteries, the anode consists of a slurry mix of silicon and graphite composite. “This new anode configuration, with silicon in it, enables manufacturers to introduce a bigger quantity of lithium in the same space, increasing the capacity of the battery”, explains Jakub Drnec, corresponding author of the publication and scientist in charge of ID31.
In order to characterise the new materials and determine their behaviour, the team first used the ILL’s instrument NEXT, as neutrons are an optimal tool to observe the distribution of lithium in the battery. At NeXT, 3D high resolution neutron tomography is coupled with X-ray tomography to image the entire cell. Subsequently they analysed the samples on beamlines BM05 and ID31 at the ESRF in operando conditions, i.e. while they were charged and discharged. In particular, they tracked Li dynamics and the morphology of the composite and how it changed over time using the technique of small angle X-ray scattering (SAXS). They also used X-ray diffraction (XRD) to study the cathode charging and absorption X-ray tomography to investigate the voids created that can lead to mechanical failure.
“This is a unique multi-modal study where we have combined neutron and X-ray tomography data from the same battery together and got a full picture of what is happening”, explains Drnec. “It shows how vast and comprehensive our research is when we use both X-rays and neutrons”, he adds.
Silicon agglomerations
The results show that the way the slurry of materials is mixed during wet electrode processing is uneven, with large silicon chunks not being mixed with graphite, and this triggers a break of chemistry around the anode. That part of the battery then becomes inactive and the defects can cause mechanical failure in the cell. “What is surprising is that after the first charge of the battery we already observe this phenomenon”, says Drnec.
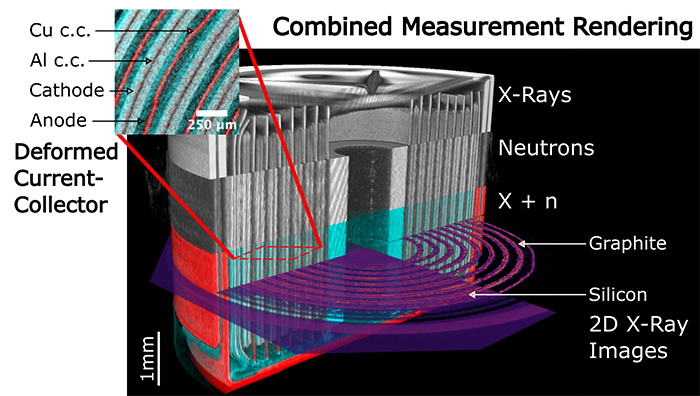 |
|
Multimodal correlative data and 3D rendering of the cell. Top part: X-ray CT data. Middle part: neutron CT data. Bottom part: Combined NXCT data in false color. A 2D SWAXS CT slice is also shown in the measured position. Right: Integrated graphite intensity. Left: Integrated SAXS intensity. The insert highlights the various components and internal cell damage as observed with NXCT. Credits: Lübke E. et al, Energy and Environmental Science, 14 May 2024. https://doi.org/10.1039/D4EE00590B |
The scientists have determined a threshold for future batteries using this composite anode: manufacturers should avoid silicon agglomerations above 50 microns, as these jeopardise the proper functioning of the battery. This thorough study provides manufacturers with clues to tweak their processes that they couldn’t have discovered without using the large research infrastructures like the ESRF and the ILL.
The research has taken place in the framework of the Battery Hub, created by the ESRF, ILL and CEA to accelerate research and innovation so that the next generation of batteries are more efficient, safer, cheaper and more sustainable.
The first author of the research is Erick Lübke, an ILL PhD student of the EU programme InnovaXN, a doctoral training programme led by the ESRF and the ILL that brings together the expertise of large-scale research infrastructures with the R&D needs of European industry.
Reference:
Lübke E. et al, Energy and Environmental Science, 14 May 2024. https://doi.org/10.1039/D4EE00590B
Text by Montserrat Capellas Espuny
Top image: An overview of the aged battery as it is being charged at the ESRF. Credits: Lübke E. et al, Energy and Environmental Science, 14 May 2024. https://doi.org/10.1039/D4EE00590B



