- Home
- News
- General News
- Visualising sperm...
Visualising sperm binding to the egg surface in 3D
16-06-2017
Scientists from Karolinska Institutet in Sweden and the ESRF have taken the first 3D snapshots of a sperm protein attached to a complementary egg coat protein at the beginning of fertilisation. The study reveals a common egg protein architecture that is involved in the interaction with sperm in both mollusc and mammals and is published in the journal Cell.
Share
The encounter between female and male gametes at fertilisation is one of the most fundamental processes in biology because it transmits the genetic information to the next generation and marks the beginning of a new life. Although egg and sperm were first observed centuries ago, how sperm recognises the coat of the egg and penetrates it has remained unknown.
Using X-ray crystallographic data collected at the ESRF's ID29 beamline, the team first visualised the sperm-interacting regions of two egg coat proteins, ZP2 and VERL. These are respectively found in mammals - including humans -, and in the marine mollusc abalone, a classic model system of invertebrate fertilisation. Remarkably, both ZP2 and VERL contain repeated sequences that play a key role in gamete recognition.
“Mammals and molluscs are thought to be separated by 600 million years of evolution, and their sperm receptor proteins are almost completely different in sequence. However, comparison of the structures of ZP2 and VERL repeats conclusively demonstrates that both proteins share a common 3D architecture”, says Luca Jovine, Professor of Structural Biology at the Department of Biosciences and Nutrition and the Center for Innovative Medicine at Karolinska Institutet.
The ESRF played an important role in the research: “The determination of the structures reported in this study was possible thanks to great performance of the ESRF beamlines”, explains Luca Jovine. The collaboration between the ESRF and Jovine’s team has been going on for many years and has always been very fruitful. Back in 2010, the team reported the structure of ZP3, another major egg coat protein from vertebrates that has also been implicated in sperm binding. However, that study did not show how the egg coat interacts with sperm at the atomic level.
Lock and key mechanism
The research group determined crystal structures of different VERL repeats bound to lysin, the counterpart protein of VERL on abalone sperm. This gave an unprecedented view of how gametes recognise each other in a species-specific way at the beginning of fertilisation.
“Abalone was our system of choice for this investigation, as it is one of the few organisms where complementary egg coat and sperm proteins are known. Moreover, fertilization in abalone is highly species-specific: even if different species of abalone spawn in the open sea and there are overlapping habitats and breeding seasons, hybrids rarely occur”, says Jovine.
The VERL-lysin complex structures also suggest how lysin opens a hole into the egg coat, allowing sperm to penetrate into the egg.
“Gamete recognition was first compared to a lock and key mechanism more than one hundred years ago. Our study provides the first example of how this is achieved at the very beginning of fertilisation”, concludes Luca Jovine.
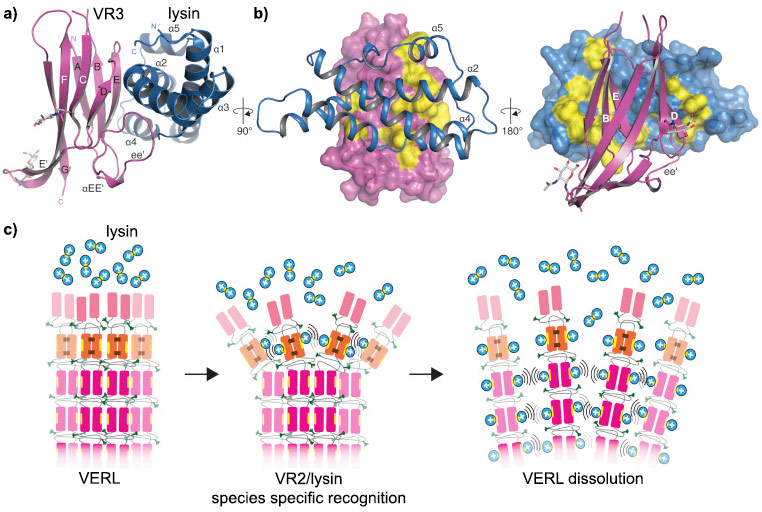 |
|
VERL/lysin interaction. a) VERL VR3/lysin complex show in cartoon representation, VR3 (dark pink) and lysin (blue). b) Surface representations of VR3 (left) and lysin (right), highlighting hydrophobic regions (yellow) at the interface with the respective partners. Main secondary structure elements involved in the interaction are marked. c) Proposed mechanism for egg coat recognition and penetration by sperm involves disruption of VERL repeat branches by lysin. Positively charged lysin molecules are represented by blue circles, with the hydrophobic VERL repeat-binding site shown as a yellow ellipse. |
Daniele de Sanctis, scientist on ID29 at the ESRF, says that “it is great to be part of this very exciting project that has already been running for many years. It is amazing to capture the molecular details of a crucial moment of the beginning of life and that the ESRF could help to illustrate it”.
Reference
Isha Raj et al., “Structural Basis of Egg Coat-Sperm Recognition at Fertilization”, Cell, online 15 June 2017. DOI: 10.1016/j.cell.2017.05.033.
The research was supported by the European Research Council (grant 260759), the Center for Innovative Medicine, the Swedish Research Council (grant 2012-5093), the Göran Gustafsson Foundation for Research in Natural Sciences and Medicine, the Sven and Ebba-Christina Hagberg foundation, and an EMBO Young Investigator award to Luca Jovine.
Top image: Credit: ugurhan.



