- Home
- News
- Spotlight on Science
- Complex patterns...
Complex patterns in liquid crystals
16-08-2011
A new way of making small molecules self-assemble into highly-complex nanopatterns has been discovered by researchers at University of Sheffield (UK) and Martin Luther University in Halle (Germany). It will help expand the capabilities of ‘bottom-up’ methods of nanopatterning for advanced functional materials through molecular self-assembly.
Share
In recent years, liquid crystals have been shown to form many diverse nanoscale structures that are significantly more complex than those found in the traditional liquid crystals used in displays. Particularly intriguing are the new liquid crystal honeycombs formed by T- or X-shaped molecules composed of several mutually-incompatible rigid and flexible parts. A bee honeycomb can be represented in 2D as a tiling patten of hexagonal tiles. Here we show how the tiles in a liquid crystal can be of many different shapes and colours, forming periodic patterns of high complexity.
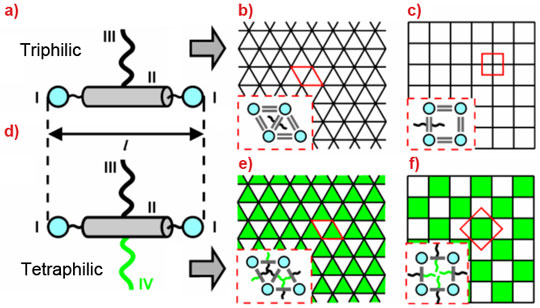
T-shaped molecules consisting of a rigid rod aromatic core (II in Figure 1a) capped at each end with hydrogen-bonding groups like glycerol (I) and having a flexible non-polar side chain, such as alkyl (III), have been found to assemble in honeycombs with cells of different polygonal cross-sections [1,2]. The rods, lying perpendicular to the channel axis, form the cell walls (“wax”), while the side-chains (“honey”) fill the cells. The larger the side-chain, the more sides to the polygon; thus compounds with small chains form triangular cells (Figure 1b), those with longer chains form square cells (Figure 1c), through pentagonal, hexagonal and beyond. If, however, a second chain (IV) is attached to the opposite side of the rod, an X-shaped molecule results (Figure 1d). If, moreover, that second chain is incompatible with the first one, we could obtain honeycombs described as two-colour tiling patterns. Two examples (triangular and square chess-boards) are shown in Figures 1e and 1f. If chain III is an alkyl and chain IV a perfluoroalkyl (Teflon), then because of their incompatibility, they will separate into alkyl (white) and fluoro (green) cells. Honeycombs in Figures 1e and 1f will form if the volumes of the two side-chains are similar. If they are not, more elaborate tiling patterns can form, such as the two-colour kagome pattern combining hexagons and triangles [3].
 |
|
Figure 1. Schematics of liquid crystal molecules and honeycombs. a) T-shaped “triphilic” molecules form “single-colour” honeycombs (b,c); d) X-shaped “tetraphiles” form “two-colour” honeycombs (e,f). |
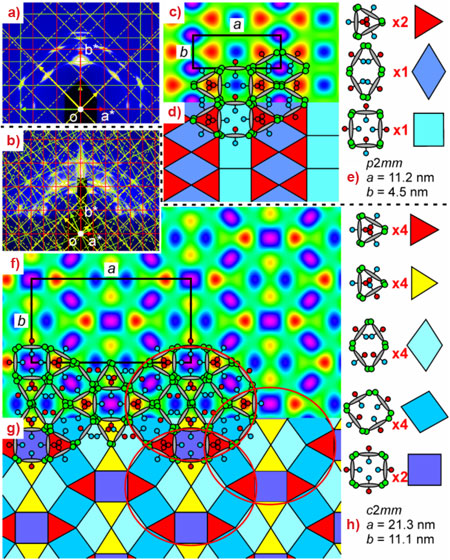
But what if the chain volumes are too large for triangular yet too small for square cells? In this case, regular structure formation can be highly frustrated (note that a square is 2.3 times larger than an equilateral triangle with the same side). The situation is further complicated if the two chains differ considerably in size. Utilising such geometric frustration, we have produced a range of LC honeycombs with highly-complex self-assembled “multicolour tiling patterns”. As part of a larger effort well-aligned thin films of X-shaped liquid crystals on silicon surfaces were studied using grazing-incidence small-angle X-ray scattering (GISAXS) on beamline BM28 (XMaS). Figures 2a and 2b show GISAXS patterns of two honeycomb phases of a compound with a perfluoroalkyl and a carbosilane side-chain. Superimposed on the GISAXS patterns are the corresponding 2D reciprocal lattices in several orientations (colour-coded), matching all observed Bragg spots. Although the molecules are small (ca 3 nm), the unit cells are large: 21 x 11 nm for the high-temperature phase. By Fourier analysis of powder patterns, electron density maps were constructed (Figures 2c and 2f). To explain the maps, schematic molecules are superposed, bearing in mind that the perfluoro chains have the highest electron density (blue-purple) and the carbosilane chains the lowest (red). No clean separation was possible between the two types of chains in either of the two phases. The optimum compromise achieved by the liquid crystal in the low-temperature phase is a honeycomb composed of pure carbosilane triangles (Figure 2e, red), pure fluoroalkyl rhombuses (dark blue), and squares in which the two chains are mixed (light blue).
Even more complex is the high-temperature phase. This honeycomb contains 18 channels in a unit cell. The channels are of five different colours (compositions) and four different shapes, shown in Figure 2h.
These structures show the remarkable ability of liquid crystals to find the optimum solution to the problem of geometric and compositional frustration, and the overwhelming tendency to form periodic structures. Even so, for even larger unit cells, a point may be reached when the system cannot maintain long-range periodicity and settles into an aperiodic structure. This still does not mean giving up on long-range order, as shown by the recently discovered soft quasicrystals with the “forbidden” twelve-fold rotational symmetry in supramolecular dendrimers [4] and three-arm star copolymers [5]. In fact, the tiling pattern in Figure 2g already shows features hinting that a dodecagonal liquid crystal honeycomb may be possible in future (see the red ellipses).
Principal publication and authors
Complex Multicolour Tilings and Critical Phenomena in Tetraphilic Liquid Crystals, X.B. Zeng (a), G. Ungar (a), C. Tschierske (b), R. Kieffer (b), B. Glettner (b), F. Liu (a), C. Nürnberger (b), K. Pelz (b), M. Prehm (b), U. Baumeister (b), H. Hahn (c), H. Lang (b), G.A. Gehring (a), C.H.M. Weber (a), J.K. Hobbs (a), Science 331, 1302 (2011).
(a) University of Sheffield, Sheffield (U.K.)
(b) Martin-Luther-University Halle-Wittenberg, Halle (Germany)
(c) TU Chemnitz, Chemnitz (Germany)
References
[1] C. Tschierske, Chem. Soc. Rev. 36, 1930 (2007).
[2] G. Ungar, C. Tschierske, V. Abetz, R. Holyst, M.A. Bates, F. Liu, M. Prehm, R. Kieffer, X.B. Zeng, M. Walker, B. Glettner, A. Zywocinski, Adv. Funct. Mater. 21, 1296 (2011).
[3] B. Glettner, F. Liu, X.B. Zeng, M. Prehm, U. Baumeister, M.A. Bates, M. Walker, P. Boesecke, G. Ungar, C. Tschierske, Angew. Chem. Int. Ed. 47, 9063 (2008).
[4] X.B. Zeng, Y. Liu, G. Ungar, V. Percec , A.E. Dulcey, J.K. Hobbs, Nature 428, 157 (2004); G. Ungar, X.B. Zeng, Soft Matter, 1, 95 (2005).
[5] K. Hayashida, T. Dotera, A. Takano, Y. Matsushita, Phys. Rev. Lett. 98, 195502 (2007).
Top image: Complex patterns in liquid crystals.