- Home
- News
- Spotlight on Science
- Fate of silver nanoparticles...
Fate of silver nanoparticles in agricultural soils amended with sewage sludge
23-05-2016
Silver nanoparticles released from consumer products end up in waste water treatment plants and may then be transferred to agricultural soils through the use of sewage sludge as fertiliser. Results from this study challenge the current idea that silver nanoparticles transform into a single, well-crystallised form of Ag2S. A combination of X-ray techniques has allowed the identification of a secondary Ag-S form (amorphous Ag2S and/or Ag-thiol) as well as nanosized mixed metallic sulfides in sludge and soil samples.
Share
Waste water treatments plants are considered as major hubs controlling the release and fate of silver nanoparticles (Ag-NPs) in the environment. Sulfidation plays a major role in the reduction of the potential impact of these nanoparticles [1]. This study confirms Ag2S as the main species in polluted sludge and amended soils. However, thanks to a combination of techniques available at the ESRF, the presence of another secondary Ag-S species and mixed metallic sulfides have also been evidenced. Despite the extremely low solubility of Ag2S, our results showed a possible interconversion between Ag2S and Ag secondary species under the influence of soil components, microorganisms and plant exudates. Nanosized Ag2S was present as heteroaggregates, Ag bound to thiol groups and/or amorphous Ag2S and nanosized mixed metallic sulfides found in this study may have very different properties to pure, macrocrystalline, Ag2S. A possible release of Ag due to the mobilisation of micronutrients by plants still needs to be evaluated. The high preferential association of Ag to the organic fraction of soils is another important insight of this work since a release of Ag is possible in the long term through organic matter turnover.
Due to their antimicrobial activity, silver nanoparticles are used in many consumer products [2] from where they are easily leached and transported by sewer systems to waste-water treatment plants. In these treatment plants, the treated water is separated from a solid phase (sewage sludge) where the pollutants, such as Ag-NPs, are retained. This sludge is rich in organic matter and nutrients and is often applied to agricultural soils as fertiliser. The aim of this work was to characterise the fate of Ag-NPs in a sludge-amended soil cultivated with crop species with agricultural interest to assess Ag-NPs impact on soil quality and crops production, and also to assess the risk of their transfer to the food chain. To this end, a polluted sludge was produced by spiking a pilot waste water treatment plant with Ag-NPs at the Swiss Federal Institute of Aquatic Science and Technology (EAWAG). The sludge was mixed with an agricultural soil and then rape and wheat were grown on the mixture at the Institut des Sciences de la Terre (ISTerre) in Grenoble.
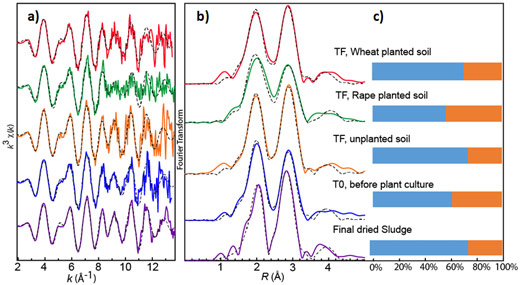
Ag K-edge bulk EXAFS spectroscopy performed at beamline BM30B (FAME) (Figure 1) confirmed that Ag2S was the main species in the sludge and amended soil before and after plant culture. However, a second Ag-S species, organic and/or amorphous Ag-S, was also identified. The proportion of this secondary species varied slightly (from 24% to 36%) depending on the sample, suggesting a possible interconversion between Ag2S and Ag secondary species under the influence of soil components, microorganisms and plant exudates. This species may have different properties to Ag2S.
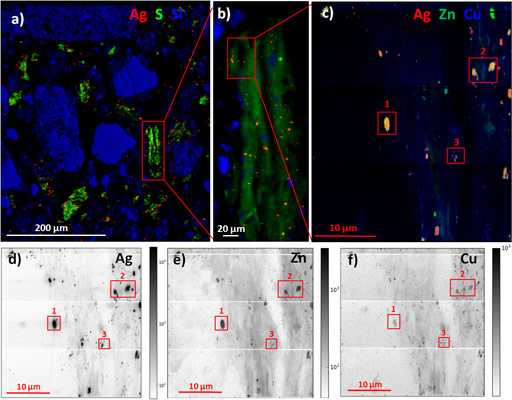
Micro X-ray fluorescence (µXRF) analyses performed at ID21 (Figure 2) showed that Ag-containing particles were well distributed throughout the sludge and soil matrix and were located in spots with size ranging from ≤0.5 to 1-3 µm. Ag formed heteroaggregates and was preferentially associated with S-rich particles, including organic fragments, with very few spots of Ag associated with Si-rich minerals. These findings have environmental implications since, on one hand heteroaggregates of nanoparticles with soil components have been shown to be better transported than homoaggregates [3] and on the other hand because the decomposition of organic matter may lead to the release of Ag-containing particles.
The extremely high sensitivity and lateral resolution of nano-XRF performed at ID16B (Figure2) also allowed us to detect nanosized Ag-containing particles, as well as diffuse and low Ag concentration in organic particles. This observation confirmed the formation of Ag bound to thiol groups of organic matter suggested by EXAFS. This species may not be stable in the long term due to organic matter turnover. This analysis also evidenced the presence of mixed metallic sulfides with Zn and Cu. The solubility and availability of Ag in these mixed sulfides is likely to be different, and possibly higher than in pure Ag2S phase. Also, as Zn and Cu are micronutrients, their mobilisation by plants could lead to the release of the Ag associated with them.
Ag availability in the pore water and DTPA (diethylene triamine pentaacetic acid) and CaCl2-extractable fractions in soils were also determined. Ag was not detectable in the pore water and very weakly exchangeable (<0.06% with DTPA and <0.04% with CaCl2). However, the presence of Ag in the soil negatively influenced wheat growth. Additionally, a negative impact on the activity of soil microorganism was also found (Pradas del Real et al. in preparation).
Together, these results have shown a more complex picture for the fate of silver nanoparticles in soils than would have been expected for simple sulfidation. Such knowledge is essential for risk assessment and legislation on the use of nanomaterials, and for safer-by-design nanomaterials, taking into account their entire life cycle.
Principal publication and authors
Fate of Ag-NPs in sewage sludge after application on agricultural soils, A.E. Pradas del Real (a,b), H. Castillo-Michel (b), R. Kaegi (c), B. Sinnet (c), V. Magnin (a), N. Findling (a), J. Villanova (b), M. Carrière (d,e), C. Santaella (f), A. Fernandez-Martinez (a), C. Levard (g), G. Sarret (a), Environ. Sci. Technol. 50, 1759–1768 (2016); doi: 10.1021/acs.est.5b04550.
(a) ISTerre (Institut des Sciences de la Terre), Université Grenoble Alpes and CNRS, Grenoble (France)
(b) ESRF
(c) Eawag, Particle Laboratory, Dübendorf (Switzerland)
(d) Université Grenoble Alpes, INAC-SCIB, Grenoble (France)
(e) CEA, INAC-SCIB, Grenoble (France)
(f) Lab Ecol Microb Rhizosphere & Environ Extrem, UMR 7265 CEA-CNRS-Aix Marseille Université, CEA Cadarache, Saint Paul Les Durance (France)
(g) Aix-Marseille Université, CNRS, IRD, CEREGE UM34, Aix en Provence (France)
References
[1] C. Levard, E.M. Hotze, B.P. Colman, A.L. Dale, L. Truong, X.Y. Yang, A.J. Bone, G.E. Brown, R.L. Tanguay, R.T. Di Giulio, E.S. Bernhardt, J.N. Meyer, M.R. Wiesner, G.V. Lowry, Environ. Sci. Technol. 47, 13440-13448 (2013).
[2] The project on emerging nanotechnologies: http://www.nanotechproject.org/
[3] E.M. Hotze, T. Phenrat, G.V. Lowry, Journal of Environmental Quality 39, 1909-1924 (2010).
Top image: Silver, sulfur and silicon distribution in soil by µ-XRF.