- Home
- News
- Spotlight on Science
- Platinum hitches...
Platinum hitches a ride with sulfur in the Earth’s crust
10-09-2021
Platinum is known to be poorly soluble and weakly mobile in aqueous solutions. However, X-ray spectroscopy studies at beamline BM30 have revealed that a particular chemical form of sulfur, the trisulfur radical ion [S3•]−, forms highly stable soluble complexes with platinum, which are capable of transporting the metal by hydrothermal fluids in the Earth’s crust.
Share
Platinum-group elements (PGE: Pt, Pd, Rh, Ru, Ir, and Os) are among the most valued metals required for new technologies; in particular, for the car industry, catalytic nanomaterials or medicine, with current prices for some of them up to 10 times higher than those of gold (e.g., rhodium, Rh >520 €/g). However, like gold, these metals are extremely rare in nature, with average tenors in terrestrial rocks of less than 1 mg per ton. How can they be concentrated in the Earth’s crust to attain mineable (i.e., economically significant) levels that must be at least 1000 times greater?
The mechanisms controlling the distribution and concentration of PGE in nature are insufficiently understood, particularly the role of aqueous fluids that circulate in the Earth’s crust and form ore deposits of other metals such as copper, zinc, silver or gold. In contrast to those metals, PGE are known to be poorly soluble in aqueous solutions containing salt (e.g., as Na+ and Cl–) and sulfur (as hydrogen sulfide, HS–, or sulfate, SO42–), despite multiple observations of enhanced PGE mobility in different hydrothermal settings within the Earth’s crust.
Researchers have attempted to solve this paradox by investigating the factors that may control the mobility of platinum, which is one of the most widely used elements of the group, in hydrothermal fluids. The most direct in-situ method available so far, synchrotron X-ray absorption spectroscopy (XAS), was used at beamline BM30 to measure both solubility and the molecular state of platinum in model hydrothermal fluids under controlled laboratory conditions similar to those in the shallow crust of the Earth (~300°C, ~2 km depth).
These unique in-situ measurements, performed using a hydrothermal autoclave designed at the Neel Institute [1], were coupled with theoretical molecular dynamics simulations that enabled the researchers to constrain the exact atomic-scale structure and composition of the species transporting platinum (Figure 1). Such fluids dominantly contain chloride, sulfate and sulfide, and, in much smaller quantities, the trisulfur radical ion [S3•]−, discovered recently [2-3], but so far ignored in models of Pt solubility in aqueous fluids.
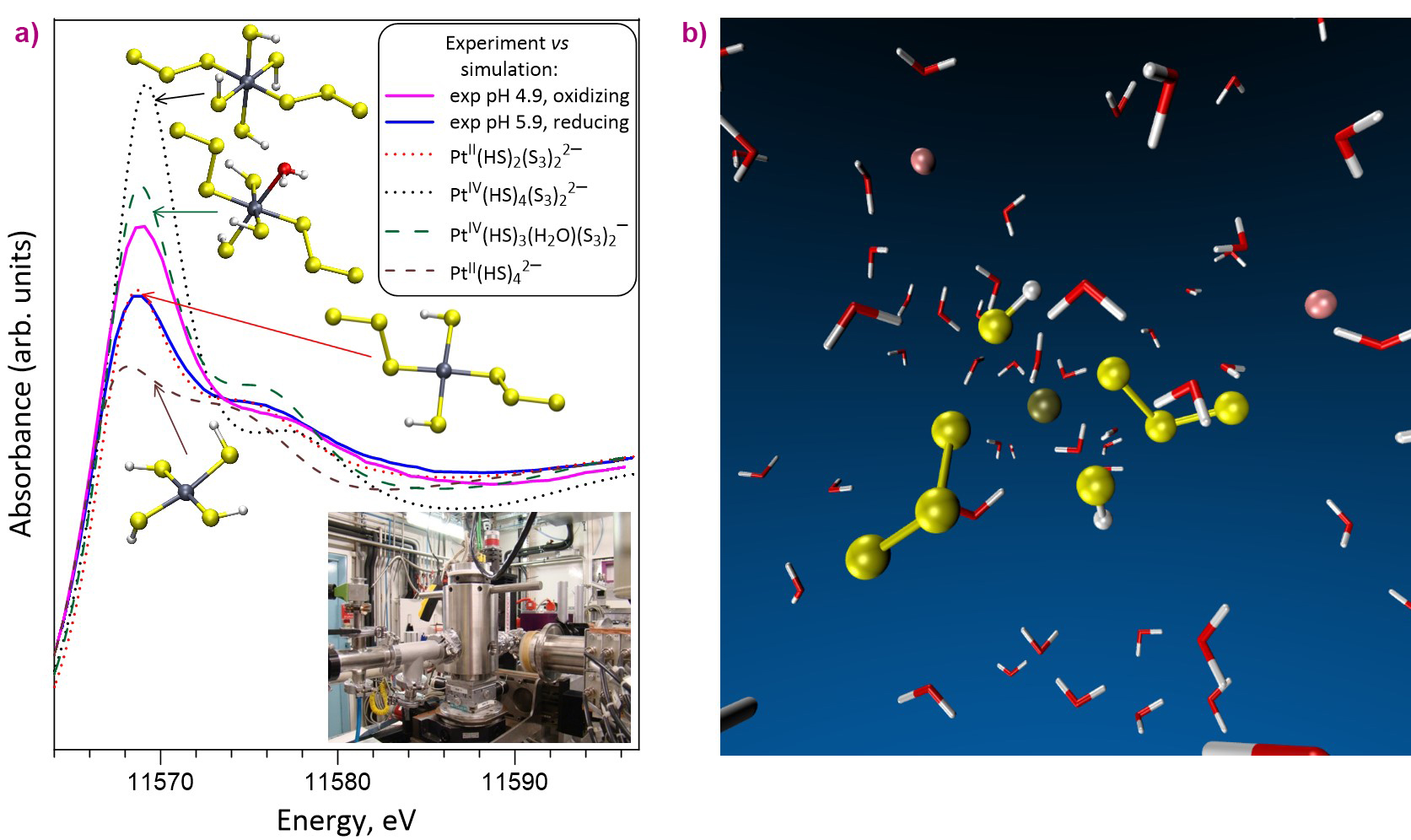
Fig. 1: a) Platinum L3-edge XANES spectra of experimental S-bearing aqueous solutions recorded at 275°C and 700 bar using the BM30 autoclave and compared with quantum-chemistry simulated XANES spectra of representative [Pt-HS-S3]-type complexes with structures optimised using molecular modelling. b) A 3D snapshot of dissolved platinum in hydrothermal fluid obtained from molecular dynamics simulations. Coupled with in-situ spectroscopy, such simulations provide information about the atomic structure and stability of chemical species in aqueous fluids that deliver critical metals to ore deposits in the Earth’s crust. Water molecules are depicted as red-and-white boomerangs. Sulfur, platinum and sodium ions are shown, respectively, as yellow, brown and pink spheres.
Unexpectedly, it was found that this minor sulfur compound binds very strongly to both Pt(II) and Pt(IV) species, thereby enabling its transport at tenors 10 000 times more than any other Cl- or S-bearing compound allows. The molecular structure and thermodynamic stability of the complexes derived in this work enables far-improved predictions of Pt solubility, transport and precipitation by aqueous fluids, both in natural and technological fluids. The chemical affinity of the [S3•]− ion for platinum (which is equivalent to the thermodynamic stability of a complex) is many orders of magnitude greater than for gold and other, more common metals [4].
This discovery may have new applications in geosciences, economic geology and nanotechnology. Acting like powerful vehicles, the trisulfur radical ions are capable of carrying tens to hundreds of grams of Pt per cubic metre of fluid, fulfilling one of the fundamental conditions of metal ore deposit formation – the presence of a metal-enriched fluid phase. Therefore, the study may offer ways to identify new potential resources of this eagerly sought metal. Furthermore, the strong selectivity of [S3•]− for Pt and, possibly by analogy, for electronically similar metals of the PGE family (e.g., Pd, Ir) could be used to improve selective extraction of those metals from ore as well as to explore new routes for PGE-based nanomaterials synthesis.
Principal publication and authors
The trisulfur radical ion S3•− controls platinum transport by hydrothermal fluids,
G.S. Pokrovski (a), M.A. Kokh (a), E. Desmaele (b), C. Laskar (a), E.F. Bazarkina (c,d), A.Y. Borisova (a,e), D. Testemale (c), J.-L. Hazemann (c), R. Vuilleumier (b), G. Ferlat (f), A.M. Saitta (f),
Proc. Natl. Acad. Sci. U.S.A. 118(34), e2109768118 (2021); https://doi.org/10.1073/pnas.2109768118
(a) Experimental Géosciences Team (GeoExp), Géosciences Environnement Toulouse (GET), CNRS, University of Toulouse (France)
(b) PASTEUR, Département de Chimie, École Normale Supérieure, Paris (France)
(c) Institut Néel and ESRF, Grenoble (France)
(d) Institute of Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry (IGEM), Moscow (Russia)
(e) Geological Department, Lomonosov Moscow State University (Russia)
(f) IMPMC, Sorbonne Université, Paris (France)
References
[1] D. Testemale et al., Rev. Sci. Instrum. 76, 043905-043909 (2005).
[2] G.S. Pokrovski & L.S. Dubrovinsky, Science 331, 1052-1054 (2011).
[3] G.S. Pokrovski & J. Dubessy, Earth Planet. Sci. Lett. 411, 298-309 (2015).
[4] G.S. Pokrovski et al., Proc. Natl. Acad. Sci. U.S.A. 112, 13484-13489 (2015).



