- Home
- News
- Spotlight on Science
- Confocal laser scanning...
Confocal laser scanning microscopy reveals trails of radiation damage in bone collagen
30-01-2023
Researchers have analysed bone radiation damage from high-energy X-rays using micrometre-resolution X-ray beams at ESRF beamline ID19 and at BESSY II synchrotron. They uncovered the destruction of collagen fibres induced by electrons emitted from mineral nanocrystals in the nanocomposite.
Share
It has long been known that, beyond a certain irradiation dose, X-rays damage living tissue. For this reason, there are clear medical recommendations to keep exposure to X-rays to a minimum. In basic research, however, when characterising mineralised tissue samples such as bone, researchers rely on increasingly powerful X-ray sources to perform tomography or diffraction measurements. Such experiments have become standard for bone research and have yielded countless insights into healthy and diseased tissues.
Until now, a prevailing concept was that the more flux and the higher the resolution, the better for the measurement. Recent advances in automated image processing and the growing availability of artificial intelligence tools make the case for achieving faster data acquisition rates, larger numbers of datapoints or greater image numbers to achieve better quality or higher resolution. For bone studies, it has become the norm to keep doses below a threshold that relies on simple assumptions of absorption per mass, thought to maintain the bony structure below a damage threshold, as has been reported in the literature. As compelling as this may appear, such simplification does not consider the composite nature of bone, where heavier, photon-absorbing elements populate mineral nanocrystals residing in a polymer mesh of collagen fibres that entain strength and toughness to bones.
In this context, a team of scientists have analysed the signals arising from various bony samples, ranging from fish to mammalian bones and teeth, using micro-computed tomographic imaging (µCT) at beamline ID19 at the ESRF and by X-ray diffraction (XRD) mapping and tomography at the BESSY II synchrotron in Berlin.
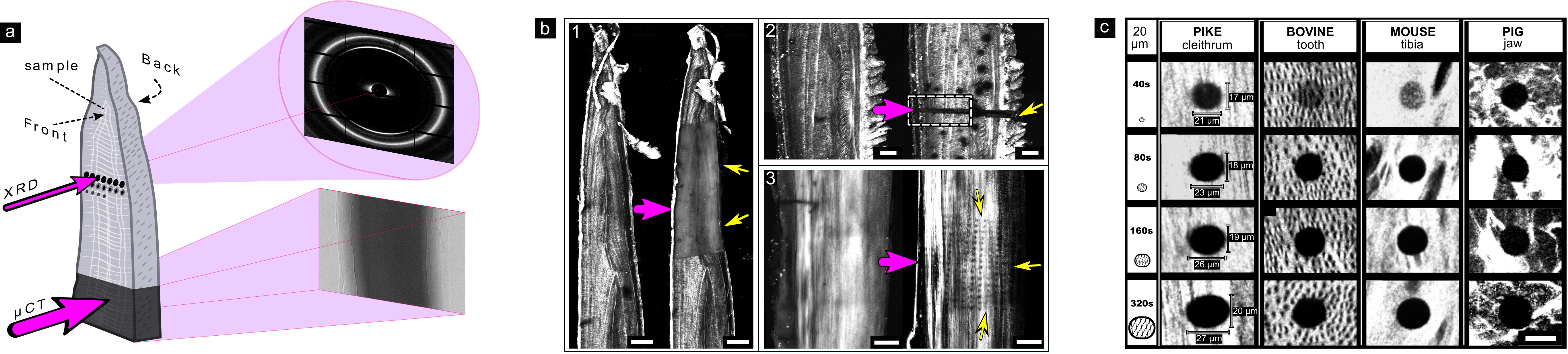
To image the protein fibres directly within the bones, the samples were examined using Second-Harmonic Generation (SHG) confocal laser scanning microscopy. These revealed the fibril layout for each bone type. When the same samples were measured using X-rays, a trail of damage was observed. Regions of damage were clearly visible in sample domains imaged tomographically using µCT at ID19 (Figure 1a), and points of diffraction irradiation performed at BESSY II (XRD and XRD-µCT) were clearly revealed by comparison of SHG images obtained before and after X-ray exposure (Figure 1b and c).
Click figure to enlarge
Fig. 1: Imprints of collagen destruction in bone collagen caused by different synchrotron experiments.
a) Schematic illustration of XRD performed at BESSY II and µCT performed at ID19 at the ESRF. b) SHG microscopy reveals collagen fibres and shows visible damage (black) to collagen (white) following measurements by: 1) µCT, 2) XRD-µCT and 3) XRD. c) Damage progression in collagen over time observed in bones of different animals, revealed by SHG.
Precise measurements of the XRD beam profile and the dimensions of damage spread revealed that, unexpectedly, the damage spreads into bone regions that were not directly exposed to the incoming X-ray beam. Measurements of strain relaxation due to damage accumulation, as well as Monte Carlo simulations and modelling, revealed the cause of damage: high-energy electrons, emitted from the crystals of bone following photon absorption, scatter in all directions, leaving the nanocrystals and gradually ionising and destroying the surrounding organic matrix. These can be explained as follows:
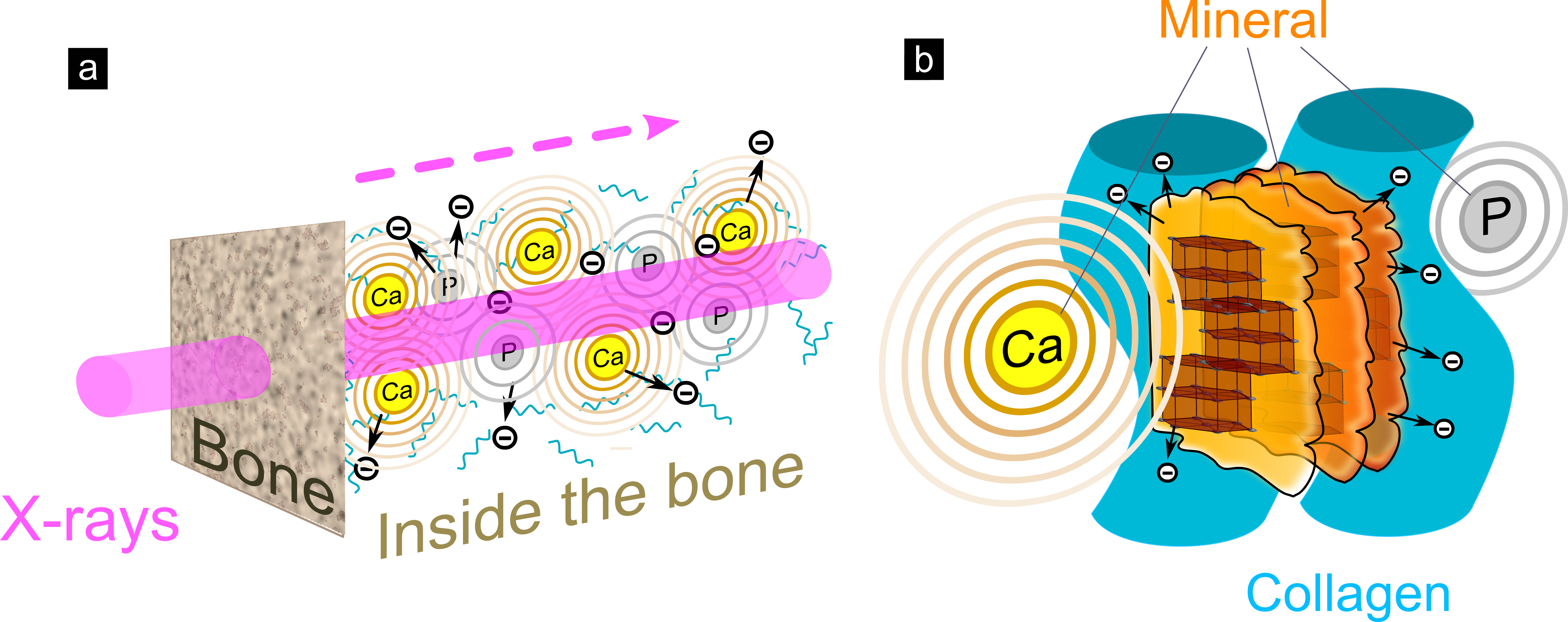
1. High-energy photons from incoming diffraction or imaging X-ray beams trigger a cascade of electron excitations (Figure 2a).
2. Ionisation of calcium and phosphorus in the mineral then damages the organic collagen framework in bone (Figure 2b).
3. Breakdown of collagen increases with the duration of irradiation but can be identified even with short exposures at high energy and flux.
Click figure to enlarge
Fig. 2: Schematic representation of radiation damage expansion in bone. a) A cascade of electron scattering (black circles with minus symbol) and X-ray fluorescence (rings) is created by the incoming X-ray beam (magenta). Calcium (Ca) emits photoelectrons as well as X-ray fluorescence that is absorbed by phosphorous (P) and collagen (cyan). The excited photoelectrons lead to collagen disintegration. b) The combined effects of lower energy X-ray fluorescence and secondary photoelectron scattering are the main contributors to collagen breakdown and primary radiation damage in bone.
These new findings support the growing concensus that measurements of bone should be limited to very short exposure times. This means that caution should be taken in basic medical research to ensure that the bone structures of interest are not damaged. X-ray methods are considered non-destructive in materials research, but, at least for research on bone tissues, this is not completely true: whereas the mineral is more or less unaffected, the adjacent organic component is not. It has been known for some time now that X-ray measurements might be damaging to genetic material entrapped in archaeological samples. The new findings show the mechanism of damage spread and highlight the vulnerability of the fibrous component as well. So, similar to clinical use in medicine, and even when there are no living tissues or DNA to damage, it comes down to using a minimal dose to get the insights that reflect the bone condition of interest. Therefore, X-ray measurements of bone require us to pay close attention to the likely effects of radiation damage.
Principal publication and authors
Primary radiation damage in bone evolves via collagen destruction by photoelectrons and secondary emission self-absorption, K. Sauer (a), I. Zizak (b), J.-B. Forien (c), A. Rack (d), E. Scoppola (e), P. Zaslansky (a), Nat. Commun. 13, 7829 (2022), https://doi.org/10.1038/s41467-022-34247-z
(a) Charité-Universitätsmedizin Department for Operative, Preventive and Pediatric Dentistry, Berlin (Germany)
(b) Helmholtz-Zentrum Berlin, Department for Structure and Dynamics of Energy Materials (SE-ASD), Berlin (Germany)
(c) Lawrence Livermore National Laboratory, Materials Science Division, Livermore, California (USA)
(d) ESRF
(e) Max Planck Institute of Colloids and Interfaces, Department of Biomaterials, Potsdam (Germany)