- Home
- News
- Spotlight on Science
- A new inverse micellar...
A new inverse micellar lyotropic liquid crystal phase
13-03-2009
A novel lyotropic liquid crystal phase with a structure based upon a hexagonal close packing of identical quasi-spherical inverse micelles has been discovered during the course of pressure-jump experiments at the ESRF.
Share
When mixed with a polar solvent, amphiphilic molecules usually self-assemble into fluid interfacial structures where polar head groups at the interface shield non-polar hydrocarbon chains from contact with the polar solvent. Many biological amphiphiles form inverse liquid crystalline phases where the interface curves towards the polar region. These molecules play important roles in cell membranes and even in man-made applications such as lipid based drug/gene delivery systems.
A number of complex three-dimensional lyotropic liquid crystal phases are already known, such as the bicontinuous cubic phases, but so far only a single example – a cubic phase of spacegroup Fd3m – of a structure based upon a close packing of inverse micelles has been found [1].
We used the high-brilliance ESRF beamline ID02 in conjunction with a soft-matter high-pressure cell developed by Prof. R. Winter, University of Dortmund, to study the high pressure lyotropic phase behaviour, and phase transition mechanisms and kinetics, in a range of hydrated lipid model membranes [2-4]. The pressure cell allows access to temperatures in the range of 0 – 65°C, and pressures of 1 bar – 5 kbar. Pressure jumps on a timescale of 7 msec can be carried out within this pressure range, either in the upwards or downwards directions. By selecting a beam energy of 17 keV, attenuation of the X-ray beam by the two 1 mm-thick diamond windows and the sample is kept to less than 40%. From the Clapeyron equation, phase transition temperatures are normally expected to increase linearly with pressure, and with a magnitude given by the ratio of the volume change ΔVm at the phase transition to the transition entropy ΔSm. For lipid membranes, the ratio dT/dp tends to lie in the range of 10 – 30°C kbar-1. Similar magnitude effects of pressure are expected for other soft matter systems (phase transitions in DNA, conformational changes in proteins). Pressure-jump experiments have a number of advantages over temperature-jump experiments: i) the final pressure equilibrates extremely quickly, with no gradients across the sample; ii) the pressure-jump can be carried out bidirectionally (either upwards or downwards); iii) pressure does not significantly alter the solvent properties. For soft matter transitions which are too fast for the millisecond apparatus, it should be feasible to extend the pressure-jump technique down to the range of 50 – 100 microseconds, by employing piezoelectric or magnetostriction actuators.
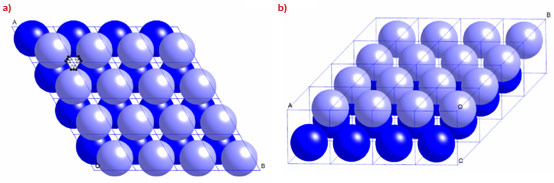
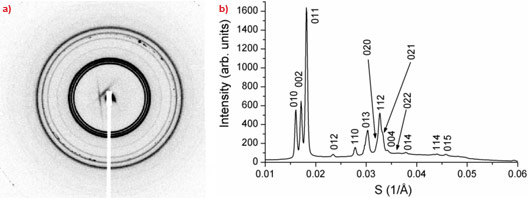
Whilst exploring the temperature-pressure-composition phase diagram of a hydrated model membrane system consisting of dioleoylphosphatidylcholine, dioleoylglycerol, and cholesterol, we found a single phase, small-angle diffraction pattern (Figure 1a and 1b), stable over a fairly wide range of temperature and pressure, which did not correspond to any known lyotropic phase. We found that these data index perfectly as 3-D hexagonal, and, using a number of additional arguments, we deduced that the structure of this phase consists of a hexagonal close packing of spherical inverse micelles, of spacegroup P63/mmc (Figure 2a and 2b).
 |
|
Figure 1. (a) SAXS pattern from new phase; (b) indexing as P63/mmc with a = 71.5 Å; c = 116.5 Å. |
Apart from its inherent academic interest in the field of soft matter self-assembly, this novel phase has a number of unique features which may render it useful for a wide range of applications. Firstly, it is the only known self-assembled lyotropic phase whose structure consists of a periodic close packing of identical inverse micelles. Secondly, it is stable in excess aqueous solution, which is very important for potential biological or biomedical applications. This new phase could have wide-ranging uses such as the storage and slow controlled release of drugs, with precise pharmacokinetics, or as nanoreactors, where the chemical or biochemical reactions would take place in identical spherical aqueous compartments having volumes as small as 10-4 attolitres (10-22 litres). It could also be used to form periodic 3-D hexagonal lattices of encapsulated nanoparticles, separated from each other by a low permittivity, 20 Å thick, fluid hydrocarbon region, which might have interesting optoelectronic properties.
References
[1] V. Luzzati, R. Vargas, A. Gulik, P. Mariani, J.M. Seddon, and E. Rivas, Biochemistry 31, 279-285 (1992).
[2] C.E.Conn, O. Ces, X. Mulet, S. Finet, R. Winter, J.M. Seddon, R.H. Templer, Phys. Rev. Lett. 96, 108102 -1 to 108102-4 (2006).
[3] J.M. Seddon, A.M. Squires, C.E. Conn, O. Ces, A.J. Heron, X. Mulet, G.C. Shearman, and R.H. Templer, Philosphical Transactions R. Soc. A 364, 2635-2655 (2006).
[4] C.E.Conn, O. Ces, X. Mulet, A.M. Squires, J. Kraineva, , R. Winter, S.M. Finet, R.H. Templer, and J.M. Seddon, Langmuir 24, 2331-2340 (2008).
Principal publication and authors
G.C. Shearman, A.I.I. Tyler, N.J. Brooks, R.H. Templer, O. Ces, R.V. Law, and J.M. Seddon, A 3-D hexagonal inverse micellar lyotropic phase, J. Am. Chem. Soc. 131, 1678-1679 (2009).
Department of Chemistry and Chemical Biology Centre, Imperial College London (U.K.)