- Home
- News
- Spotlight on Science
- Molecular mechanism...
Molecular mechanism of bacterial survival against antimicrobial peptides revealed by X-ray studies
04-08-2009
Lipopolysaccharides form the outer-most surface of Gram-negative bacteria, acting as the protector against chemical attack. Scientists have designed a model of bacterial surfaces at the air/water interface and solid/liquid interface, and then studied the impacts of ion species and cationic antimicrobial peptides on their vertical structures using grazing incidence X-ray scattering and high energy specular X-ray reflectivity.
Share
Lipopolysaccharides (LPSs) are a major component of the outer leaflet of the outer membrane of Gram-negative bacteria, which stabilise the structural integrity of bacteria, and protect the membrane against chemical and biochemical attack. Cationic antimicrobial peptides (CAPs) have been gaining attention from the food industry and medical sciences as antimicrobial alternatives to chemical food preservatives and commonly used antibiotics. Many in vivo studies demonstrated that divalent cations (Ca2+, Mg2+) significantly increase the survival rate of bacteria. However, despite some theoretical approaches that account for the role of electrostatics, the structural evidence from experimental approaches at the molecular level is still missing.
In one of our recent accounts [1], we designed a realistic model of bacterial outer membranes at the air/water interface. Here, an insoluble monolayer of lipopolysaccharide from Salmonella enterica sv. MinnesotaRa (LPS Ra) is spread on buffered subphase with and without Ca2+. Among LPS molecules from various rough mutants, the molecular structure of LPS Ra is closest to that of wild type LPS that has polydisperse O-polysaccharide chains. As a CAP, we selected herring protamine, a cationic peptide (isoelectric point pI = 10 – 12) extracted from the sperm cells used for food preservation.
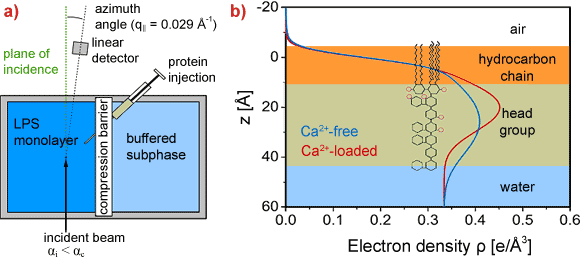
To study the conformational change of carbohydrate chains due to Ca2+, the electron density profiles normal to the surface are quantitatively reconstructed from grazing incidence diffuse X-ray scattering out of the specular plane (GIXOS) carried out at beamline ID10B. In the GIXOS measurements, the intensity of the scattered beam is collected with a position sensitive linear detector perpendicular to the monolayer surface at an azimuthal angle near the incidence plane (Figure 1a). For a very small in-plane momentum transfer (q|| ~ 0) and conformal interface roughness, the measured diffuse intensities are related to the corresponding reflectivity curve [2]. GIXOS at the air/water interface offers a unique advantage over the specular reflectivity technique by significantly reducing the irradiation time (by a factor of 100). In addition to the X-ray scattering experiment we have carried out Monte Carlo simulations to simulate the conformational change of > 102 LPS molecules over > 10–3 s, and we created a minimal computer model of LPS molecules [3].
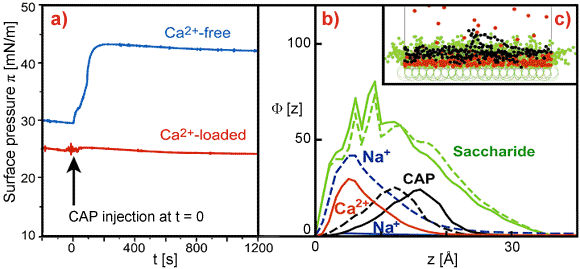
Vertical electron density profiles (Figure 1b) reconstructed from GIXOS signals imply the “collapse” of saccharide chains in the presence of Ca2+, suggesting that Ca2+ bridges the negatively charged saccharide units (pink circles in Figure 1b). Under these conditions, the LPS monolayer remains intact even after injection of protamine near the minimum inhibitory concentration (Figure 2a, red). Monte Carlo simulations confirmed that the presence of Ca2+ in the LPSs prevents the approach of CAP to the hydrophobic region. In contrast, the insertion of CAP in the absence of Ca2+ results in the complete destruction of the layered structure of LPS Ra monolayers (Figure 2b, blue).
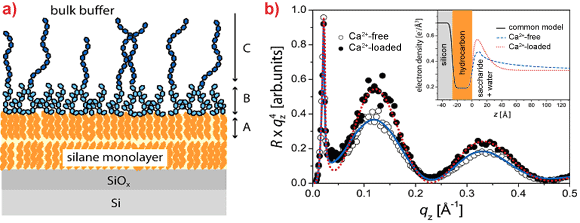
In a second study [4], we created a more complex model by deposition of monolayers of LPS from Pseudomonas aeruginosa strain dps 89 (mutant of the PAO1 strain with fully charged O-side chains) onto silicon substrates coated with alkylsilane monolayers that mimic the inner leaflet of the outer membrane of bacteria (Figure 3a). Here, the influence of ions on the out-of-plane structures was investigated by specular reflectivity with 22 keV X-rays, which guarantees high transmittance through 2 cm of water. The measurements were taken at the liquid-solid interface. Since polydisperse O-side chains are grafted with a low surface density (approx. 10%), we used a stretched exponential decay function to account for the continuous decay of the electron density towards the bulk buffer.
The electron density profiles reconstructed from the reflectivity curves (Figure 3b) and Monte Carlo simulations indicate that the presence of Ca2+ ions induces a significant (~ 20%) increase in the electron density of the core saccharide. Furthermore, the electron density of the O-side chain region decays more rapidly to the level of the bulk buffer at z > 10 Å, which coincides with a significant increase in the stretching exponent and a decrease in the decay length by a factor of more than two. Thus, our results reveal that the condensation of divalent ions (Ca2+) in the negatively charged core saccharides and a significant collapse of saccharide chains are the principal mechanisms behind the resistance of bacteria to cationic antimicrobial peptides.
References
[2] S. Mora, J. Daillant, D. Luzet, B. Struth, Europhys. Lett. 66, 694 (2004).
[3] D.A. Pink, L.T. Hansen, T.A. Gill, B.E. Quinn, M.H. Jericho, T.J. Beveridge, Langmuir 19, 8852 (2003).
Principal publications and authors
[1] R.G. Oliveira (a,b), E. Schneck (a,c), B.E. Quinn (d,e), O.V. Konovalov (f), K. Brandenburg (g), U. Seydel (g), T. Gill (e,h), C.B. Hanna (e,i), D.A. Pink (d,e), and M. Tanaka (a,c,e), Physical Mechanisms of Bacterial Survival Revealed by Combined Grazing-Incidence X-ray Scattering and Monte Carlo Simulation. Comptes Rendus Chimie Festschrift for P.G. DeGennes 12, 209-217 (2009);
[4] E. Schneck (c), E. Papp-Szabo (j,e), B. E. Quinn (e,d), O.V. Konovalov (f), T.J. Beveridge (j,e), D.A. Pink (e,d), and M. Tanaka (c,e), Calcium Ions Induce Collapse of Charged O-Side Chains of Lipopolysaccharides from Pseudomonas Aeruginosa, J. R. Soc. Interfaces 12, in press (doi: 10.1098/rsif.2009.0190).
(a) Technical University Munich, Garching (Germany)
(b) currenty at: CIQUIBIC-UNC, Córdoba (Argentina)
(c) University of Heidelberg, Heidelberg (Germany)
(d) St. Francis Xavier University, Antigonish (Canada)
(e) Network of Canadian Centre of Excellence (Canada)
(f) ESRF
(g) Research Center Borstel, Borstel (Germany)
(h) Dalhousie University, Halifax (Canada)
(i) Boise State University, Boise (USA)
(j) University of Guelph, Guelph (Canada)