- Home
- Users & Science
- Find a beamline
- Structural biology
- Our beamlines
- Cryobench laboratory
- Methods
- UV/vis Absorption
UV/vis Absorption
Overview
With our microspec setup, it is possible to record UV/vis absorption spectra of protein crystals as small as 10 μm. In fact, due to the very high optical density of protein crystals, it is often complicated to obtain spectra of crystals which are thicker than about 50 μm, as absorption bands tend to become saturated.
By comparing such crystal absorption spectra with spectra of the protein in solution (e.g., recorded on the same setup by freezing the solution in a nylon loop), it is possible to evaluate the protein's intactness in the crystalline form. If, for example, a protein's cofactor(s) are lost or altered during crystallisation, this can be reliably detected based on UV/vis absorption spectra.
Experimental setup

The two opposing objectives are used for focussing white light onto the sample and collecting the transmitted portion, respectively.
The setup is described in more detail in a paper by Royant et al. [J. Appl. Cryst. 40, 1105-1112 (2007)].
Software
Our spectrometers (HR2000 and HR2000+ from Ocean Optics) are used with the software SpectraSuite. A manual for SpectraSuite is available here.
Example
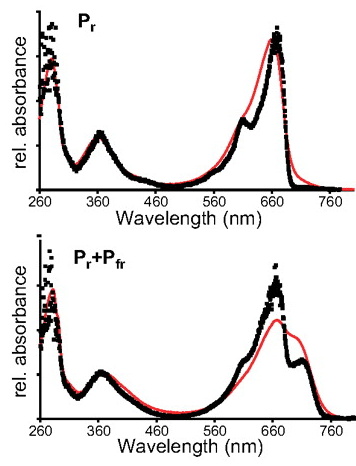
Essen et al. [Proc. Nat. Acad. Sci. 105, 14709-14714 (2008)] have used this setup to record UV/vis absorption spectra of phytochrome crystals. Phytochromes are photosensor proteins which can be reversibly switched between two stable states (called Pr and Pfr) by illumination with red and far-red light, respectively.
As shown in this figure, the crystal spectra (black squares) differ somewhat from solution spectra (red line). This is partially due to the fact that the solution spectra were recorded at room temperature, while the crystal spectra were recorded at 100 K, but the spectra are, nevertheless, very similar.
The bottom spectra, which were obtained after red illumination at room temperature, show that even some photoconversion occurs in the crystalline state.