- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2002
- Materials Science
- X-ray Diffraction of High-Pressure Fluids in Diamond-anvil Cells
X-ray Diffraction of High-Pressure Fluids in Diamond-anvil Cells
The study of the structure of liquids at high pressure and temperature has been a long-standing goal in high-pressure research. With the maturation of third-generation synchrotron sources this goal is now attainable as demonstrated by the recent observation of a first-order liquid-liquid phase transition in phosphorous in a large-volume press [1]. We have successfully extended X-ray diffraction measurements in liquids to diamond-anvil cell (DAC) experiments.
Most of the recent work on the structure of liquids at high pressure and temperature has been done on solids (at ambient conditions) like phosphorous [1]. It was our desire to study liquids and gases (at ambient conditions) such as argon, H2O, O2, and CO2. These samples can be easily loaded into a DAC but are very difficult to load in large volume presses. In order to analyse our DAC liquid diffraction data we found it possible and necessary to directly determine the density from the diffraction measurements. Thus, our experiments yielded both structural and equation-of-state information.
The thick diamond cells comprising the pressure vessel, the small sample size in DAC experiments, and the small scattering intensity of low-Z materials make these studies extraordinarily difficult. Thus, we developed a self-consistent, corrective procedure that addressed problems associated with the normalisation of the diffraction intensity, variations of the molecular form factor, and temperature diffuse scattering. Adequate attention must be paid to all of these uncertainties in order to obtain quantitative measurements of the structure factor of high-pressure fluids. A necessary component of our analysis is the determination of the density.
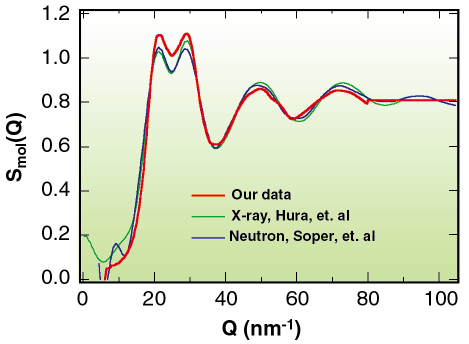
In order to ensure that the results of our experiments represented accurate results, we performed an extensive comparison of our method with high-quality determinations of the structure factor of water at ambient conditions by X-ray and neutron diffraction. This comparison is shown in Figure 85. All of the peak positions are in good agreement and the peak intensities are very nearly so. The small discrepancy in the relative and total intensities of the primary doublet at 20 and 30 nm-1 is entirely consistent with a small (< 0.05 GPa) residual pressure according to extensive high-pressure experiments.
 |
|
Fig. 85: Determination of the molecular structure factor of water at ambient conditions in a DAC compared to other ambient condition experiments. |
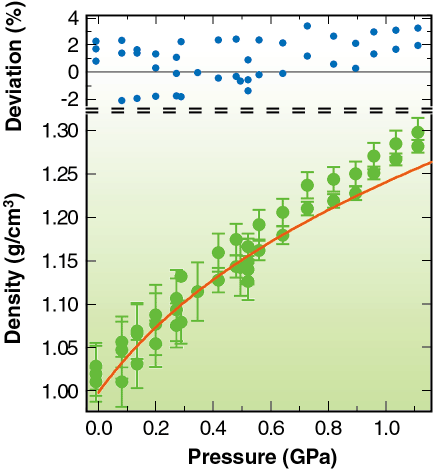
In Figure 86 we plot our determination of the density for liquid water at 295 K versus pressure. The excellent agreement between our measurements and the Saul and Wagner equation of state (shown by the red line) suggests that our analysis rests on a sound foundation, and that it is possible to measure the density of low-Z liquids by X-ray diffraction to ~ 3 % accuracy in diamond-anvil cells. This is the first time that high-pressure density measurements of liquids have been measured by X-ray diffraction, and it opens exciting new possibilities for directly measuring the equation of state of liquids.
 |
|
Fig. 86: Determination of the density of water at 295 K up to the freezing pressure. The Saul and Wagner EOS is shown by the red curve and the deviation shows that we achieve about 3% accuracy. |
In conclusion, we have measured the structure factor of liquids at high pressure in a diamond anvil cell for the first time. We have also measured the density of a liquid at high pressure by diffraction for the first time. We compared our results with high-accuracy measurements at ambient condition and to a well-known equation of state and found very good agreement.
Reference
[1] Y. Katayama, T. Mizutani, W. Utsumi, O. Shimomura, M. Yamakata, and K. Funakoshi, Nature 403, 170 (2000).
Principal Publication and Authors
J.H. Eggert (a), G. Weck (b) , P. Loubeyre (b) , M. Mezouar (c), Phys. Rev. B 65, 174105 (2002).



