- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structure of materials
- Reversible formation of a PdCx phase in Pd nanoparticles upon CO and O2 exposure
Reversible formation of a PdCx phase in Pd nanoparticles upon CO and O2 exposure
Palladium nanoparticles are usually assumed to be in a purely metallic state during a CO oxidation reaction when CO is in excess. In this work, we tested this hypothesis studying the behaviour of Pd nanoparticles under reaction conditions. A combination of X-ray diffraction and X-ray photoelectron spectroscopy was used for pressures ranging from UHV to 10 mbar and from room temperature to a few hundred degrees Celsius. Under CO exposure, above a certain temperature, carbon dissolves into the particles forming a carbide phase. Upon exposure to CO and O2 mixtures, the carbide phase forms and disappears reversibly, switching at the stoichiometric ratio for CO oxidation. We have characterised the reversible formation of PdCx in situ by monitoring the position of the Pd(111) powder ring using a 2D detector, while mixtures of CO and O2 were flown through the reactor. The size-monodisperse Pd nanoparticles (15 and 35 nm) deposited on SiOx and alumina samples were synthesised in Lund.
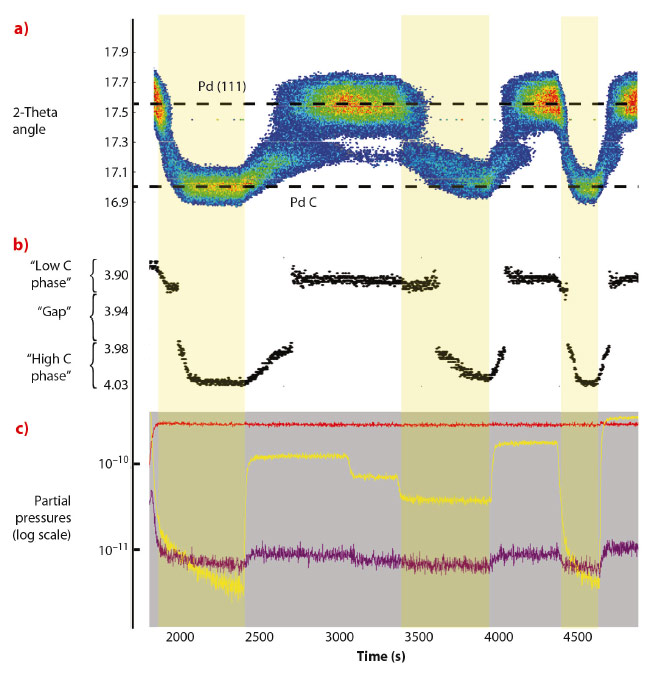
The experiment was performed at 550 K and at a constant CO pressure of 7 mbar while varying the O2 pressure from 0 to 10, 5, 2, 15, 0 and 30 mbar. The total pressure was kept constant at 1000 mbar by adding argon. Figure 112a shows the angular position of the intensity maximum in the Pd(111) region against time. Depending on the gas composition, the angular position of the Pd(111) ring changes between 17.6 degrees and 17.0 degrees (energy: 18 keV), corresponding to an expansion of the lattice constant from 3.90 Å to 4.03 Å (Figure 112b). Figure 112c shows the evolution of the gas composition in the UHV chamber. The lattice expansion is consistent with the formation of PdCx. In Figure 112b the lattice parameter of the Pd nanoparticles, extracted from the angular position of maximum intensity in the Pd(111) ring, is plotted against time. A jump can be seen in the lattice parameter evolution between 3.92 Å and 3.98 Å, which is a signature of a phase transition. The measurements demonstrate that two different carbon containing Pd phases exists depending on the gas surroundings, one with a C concentration of 1-2% and one with C concentration in the range 11-16%.
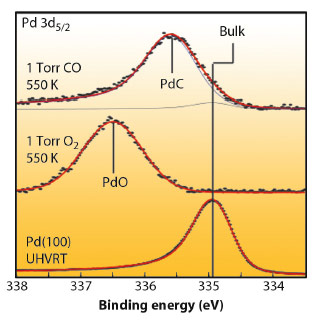
Ambient pressure XPS studies, carried out at the ALS (Beamlines 11.0.2 and 9.3.2) revealed that the Pd 3d5/2 peak shifted up, from the metallic Pd energy by 1.6 eV upon exposure of the nanoparticles to 1 mbar oxygen at 550 K, indicating the formation of an oxide shell of at least 1 nm thick. By introducing 1 mbar of CO, PdO is reduced. While a weak Pd bulk component at 335 eV appears, a new intense component can be observed at 335.6 eV (Figure 113). At these excitation energies, at most 40% of the total intensity of the peak derives from the particle surface, confirming the formation of a PdCx phase in the particle.
 |
|
Fig. 113: Pd 3d5/2 core level spectrum from a clean Pd(100) reference (bottom), after oxidation of 15 nm Pd particles at 1 mbar O2 exposure at 550 K (centre) and after 1 mbar CO exposure at 550 K (top). |
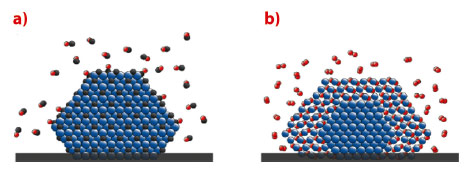
In conclusion, Pd particles will transform into PdCx (Figure 114a) in pure CO or a mixed O2/CO atmosphere with excess CO at temperatures of around 550 K, while a PdO shell forms (Figure 114b) in excess oxygen. The formation of PdCx is easier than expected, and might play an important role for palladium catalysts whenever CO is present. Our study indicates that compound formation between the active catalyst and the gas phase may be a general phenomenon.
 |
|
Fig. 114: Schematic model of a) a PdCx particle in CO pressures in the mbar range with pCO/pO2 > 2, and elevated temperatures, and b) a PdO particle with a metallic core in O2 pressures in the mbar range, pCO/pO2 < 2, and elevated temperatures (Pd in blue, C in Black, O in red). |
Principal publication and authors
O. Balmes (a), A. Resta (a), D. Wermeille (a), R. Felici (a), M.E. Messing (b), K. Deppert (b), Z. Liu (c), M.E. Grass (c), H. Bluhm (d), R. van Rijn (e), J.W.M. Frenken (e), R. Westerström (f), S. Blomberg (g), J. Gustafson (g) and E. Lundgren (g), Phys. Chem. Chem. Phys. 14, 4796-4801 (2012).
(a) ESRF
(b) Solid State Physics, Lund University (Sweden)
(c) ALS, Lawrence Berkeley National Laboratory, Berkeley (USA)
(d) Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley (USA)
(e) Kammerlingh Onnes Laboratory, Leiden University (The Netherlands)
(f) Surface Physics Group, University of Zürich (Switzerland)
(g) Division of Synchrotron Radiation Research, Department of Physics, Lund University (Sweden)