- Home
- News
- Spotlight on Science
- Spheres becoming...
Spheres becoming worms: elucidating the transformation of surfactant micelles
26-09-2014
The morphological transformation pathways in micellar systems are still not well understood despite over a century of research in surfactant science. This is mainly due to lack of sufficiently fast methods that capture structural changes underlying such transformations. In the present work, an archetypical, simple surfactant system consisting of sodium dodecyl sulphate in aqueous salt solutions was investigated using time-resolved small-angle X-ray scattering (SAXS) combined with stopped-flow rapid mixing. The results show that upon rapid change in salt concentration, “worm-like” micelles are formed by the fusion of globular and transient elongated micelles on a millisecond time scale in a similar fashion to a step-like polymerisation process.
Since their discovery over a century ago, surfactant micelles have been a topic of intense interest in both academic and technological research [1]. Surfactant molecules consist of a hydrophilic head group covalently linked to a hydrophobic tail (hydrocarbon) group. In aqueous solution above a critical micellar concentration (cmc), they self-assemble into micelles consisting of a hydrophobic core of tail groups surrounded by a shell of hydrophilic head groups and water. The shape and size of these micelles depend on the type of surfactant and thermodynamic conditions such as temperature, solvent composition and salinity. Typically surfactant micelles aggregate into highly dynamic nanostructures consisting of either spherical or non-spherical shapes such as ellipsoidal, cylindrical and discoidal shapes. Detailed insight into their fundamental behaviour is important owing to their widespread applications ranging from detergents and additives in personal care and household cleaning products to advanced applications in biotechnology and nanotechnologies. Not much is known about the dynamics and non-equilibrium kinetics associated with their different morphological transitions.
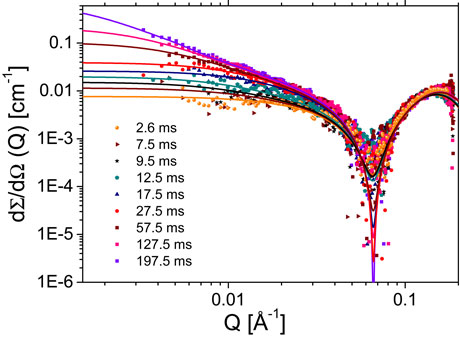
Recently, we have succeeded in following the complete kinetics of micelle formation from a pool of monomers to globular micelles using time-resolved SAXS combined with stopped-flow mixing [2]. Relatively weak structural signals over the solvent background, coupled with rapid kinetics in the millisecond range make obtaining accurate kinetic SAXS data on these systems a challenge [3]. Here we have exploited the high brilliance and low instrumental background of the ID02 SAXS instrument to map the pathways of a well-known shape transformation from spherical to worm-like micelles in a prototypical surfactant sodium dodecyl sulphate (SDS) upon the addition of NaCl. Millisecond time resolution was obtained by employing a stroboscopic sequential data acquisition scheme [3]. Figure 1 depicts the typical evolution of background-subtracted normalised SAXS intensities upon mixing solutions of 1 weight % of SDS with 2 M NaCl, corresponding to a final salt concentration of 1 M. At this salt concentration, worm-like micelles form spontaneously. This transformation can be understood in terms of the screening of the strong electrostatic repulsion between the surfactant head groups in the presence of NaCl. Thermodynamically, the reduced effective volume of head groups promotes a more elongated morphology that can be characterised in terms of the so-called packing parameter of surfactant molecules. At lower salt concentration (c.a. 0.5 M), micelles become more elongated but do not form worm-like chains.
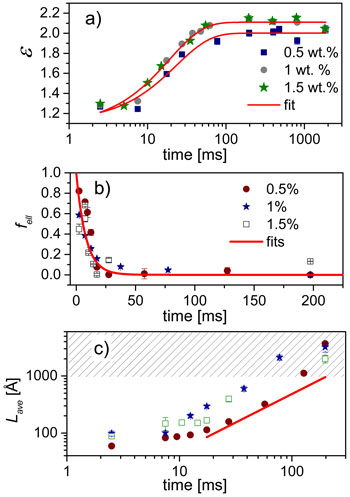
Quantitative analysis of the SAXS data in terms of a core-shell model yields the shape as well as the average size parameters of the micelles. Figure 2 presents the time evolution of the shape parameters for different SDS and NaCl concentrations. The initial SDS micelles have an ellipsoidal shape characterised by an aspect ratio (ε, the ratio of major and minor radii). At low salt concentration (0.5 M), the aspect ratio increases roughly as an exponential function but the micelles remain globular as shown in (a). At higher salt concentration (1 M), the SAXS data are best described by a linear combination of ellipsoidal and worm-like micelles. Interestingly, the fraction of ellipsoids (fell) decreases rapidly with time as depicted in (b). Concomitantly, the contour length of worm-like micelles (Lave) increases linearly as indicated in (c) and finally exceeds the resolution limit of the SAXS configuration.
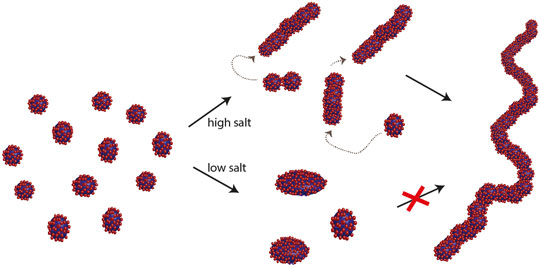
The above findings suggest that individual micelles undergo successive fusion events resulting in elongated structures. Because of the high curvature of elongated micelles, the surface energy is highest at the end of the cylinder and thus fusion events here might have higher probability. This mechanism bears similarity with step-wise growth polymerisation reactions (e.g. acid catalysed condensation polymers) where the polymers grow in a step-wise manner through addition reactions involving the end groups, hence monomers are consumed rapidly and polymeric growth occurs through combination of oligomers rather than by continuously adding monomers. A schematic illustration of the proposed mechanism is depicted in Figure 3.
 |
|
Figure 3. Schematic illustration of the transformation of spherical/ellipsoidal micelles to worm-like micelles at higher salt concentration. |
In conclusion, we have successfully followed the formation of worm-like/polymer-like micelles from globular micelles by employing time-resolved SAXS. Through a quantitative analysis, we deduced that the kinetic pathway of formation involves successive fusion events reminiscent of the step-wise polymerisation process. The identification of a polymer type growth process in surfactant solutions provides an interesting synergy in terms of a broader understanding of non-equilibrium processes in soft matter [4]. The work thus provides a unique insight that is important for both fundamental research and technological applications.
Principal publication and authors
G.V. Jensen (a), R. Lund (b), J. Gummel (c), T. Narayanan (c), and J.S. Pedersen (a), Angew. Chem. Int. Ed. 53, 1-6 (2014); DOI: 10.1002/anie.201406489.
(a) Department of Chemistry and Interdisciplinary Nanoscience Center (iNANO), Aarhus University, Aarhus (Denmark)
(b) Department of Chemistry, University of Oslo (Norway)
(c) ESRF
References
[1] Surfactant Science and Technology: Retrospects and Prospects, L.S. Romsted (Ed), CRC Press (2014).
[2] G.V. Jensen, R. Lund, J. Gummel, M. Monkenbusch, T. Narayanan, and J.S. Pedersen, J. Am. Chem. Soc. 135, 7214 (2013).
[3] T. Narayanan, J. Gummel, and M. Gradzielski, Advances in Planar Lipid Bilayers and Liposomes 20, 171 (2014).
[4] R. Lund, L. Willner, D. Richter, P. Lindner, and T. Narayanan, ACS Macro Lett. 2, 1082 (2013).
Top image: Surfactant micelles transform from spheres into worms at high salt concentrations.