- Home
- News
- Spotlight on Science
- Spinodal decomposition...
Spinodal decomposition governs the formation of the multi-scale structure of the skeletal elements of the brittle star
05-11-2019
The hierarchical structure of a brittle star’s skeletal parts has been revealed using a combination of high-resolution X-ray characterisation techniques and electron microscopy. This previously unknown structure unveils one of Nature’s strategies to create efficient and intricately-designed crystal structures.
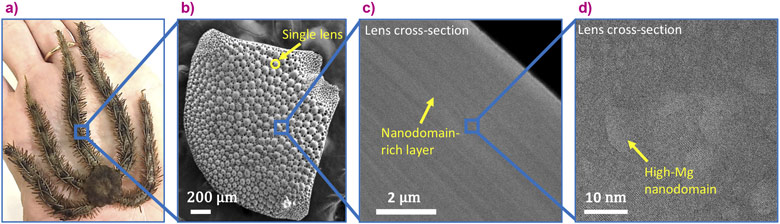
Minerals that are produced by living organisms often demonstrate fascinating structures, associated with excellent properties. This is the case of the skeletal parts of the Ophiocoma wendtii brittle star, which possesses several skeletal parts including lenses, spicules and teeth that are all made of large single crystals of calcite (a crystalline polymorph of calcium carbonate). The calcite from these skeletal parts was shown in an earlier study to be several times tougher than geological calcite [1] and that the higher toughness is due to the special structure of the brittle star’s calcite single crystals.
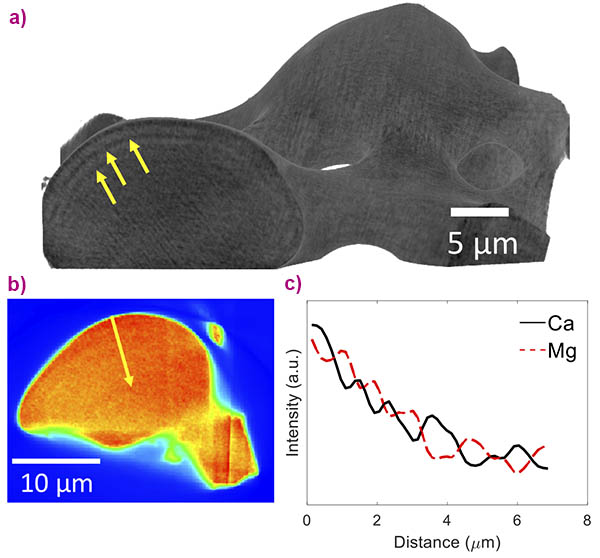
The brittle star’s calcite single crystals are intricately structured on several levels. Firstly, this natural calcite is not pure calcium carbonate but contains magnesium ions that substitute for calcium ions in the crystal lattice, as was quantified with the help of high-resolution X-ray powder diffraction performed at beamline ID22 [1]. Secondly, the Mg ions are heterogeneously distributed in the crystal lattice, and high-Mg nano-domains exist within the single crystal, as was determined via in situ heating X-ray powder diffraction performed at ID22 and via high-resolution transmission electron microscopy, performed at the Technion-Israel Institute of Technology [1]. Thirdly, the high-Mg nano-domains are heterogeneously dispersed in the crystal lattice; they are organised in layers with alternating concentrations (Figure 1). The latter was determined via nano-holotomography and X-ray fluorescence tomography, both performed at beamline ID16B, and high-resolution scanning and transmission electron microscopy, performed at the Technion.
The combination of these high-resolution techniques has allowed this thorough multi-scale characterisation of the hierarchical structure of the brittle star’s calcite single crystals. The experiment at ID16B notably combined the 3D reconstruction of a single lens, using nano-holotomography at a pixel size down to 35 nm, with chemical mapping of the lens using X-ray fluorescence tomography. The observed structural layers were revealed to be caused by the layered distribution of Ca and Mg present in the material by this cutting-edge technique (Figure 2).
This distribution of magnesium ions is the key to the material’s excellent mechanical properties. Indeed, the stress induced by the presence of the high-Mg nanodomains and the stress fluctuations induced by their layered distribution prevent crack propagation in the material. Following this full characterisation of the material, possible mechanisms of formation have been investigated for this intricate structure that is formed by a sea organism at ambient conditions. By combining results from the literature, mathematical modelling and bio-inspired experimental results on synthetic calcite (characterised at beamline ID22), the brittle star’s calcite was revealed to form via the crystallisation of Mg-rich amorphous calcium carbonate with a concentration of Mg that is in an instability region. Because of its instability, the Mg-rich amorphous calcium carbonate undergoes a spinodal decomposition leading to the formation of high-Mg nanodomains within a Mg-depleted matrix. When the amorphous calcium carbonate crystallises, the advancing crystallisation front alternatingly embeds and rejects the high-Mg nanoparticles, leading to the formation of a single crystal comprising an alternating layered structure.
This study has unveiled not only a biomineral single crystal's hierarchical structure and its formation mechanism, but also a fundamental property of Mg-rich amorphous calcium carbonate: the existence of an instability range that leads to a spinodal decomposition and the formation of high-Mg nanodomains. These results are significant for the understanding of the structure of biominerals and the mechanism of their formation. Moreover, this study opens up new synthetic routes and processes for the design of bioinspired efficient materials and structures.
Principal publication and authors
From spinodal decomposition to alternating layered structure within single crystals of biogenic magnesium calcite, E. Seknazi (a), S. Kozachkevich (a), I. Polishchuk (a), N.B. Stein (a), J. Villanova (b), J.P. Suuronen (b), C. Dejoie (b), P. Zaslansky (c), A. Katsman (a), B. Pokroy (a), Nature Communications 10, 4559 (2019); doi: 10.1038/s41467-019-12168-8.
(a) Technion-Israel Institute of Technology, Haifa (Israel)
(b) ESRF
(c) Charité–Universitätsmedizin Berlin (Germany)
References
[1] I. Polischuk, et al., Science 358, 1294-1298 (2017).