- Home
- News
- Spotlight on Science
- Characterising amyloid...
Characterising amyloid plaques in Alzheimer’s disease
14-06-2021
Researchers have analysed Alzheimer’s disease brain samples at beamlines ID21 and ID16A, utilising micro-FTIR and X-ray fluorescence microscopy to characterise two types of amyloid plaques with differences in metal ion composition and in their levels of oxidised lipids. The results could lead to new insights into the development of the pathology.
Alzheimer’s disease was first described by Alois Alzheimer in 1906 as an accumulation of an unidentified substance in the brains of people affected by dementia. It wasn’t until 1987 that the amyloid peptide was described as the main component of these deposits, known as amyloid plaques. Amyloid peptide is processed in the extracellular space in the brain and tends to aggregate, forming oligomers that further develop into amyloid fibrils in a nucleation-dependent polymerisation process. Whereas the toxicity of fibrils is known to be low, oligomers and other types of aggregates, which form under specific physicochemical conditions (low pH, metal ions, oxidised lipids) are known to be toxic to cells [1]. The fact that these toxic amyloid aggregates have, until now, mainly been described in vitro, makes their identification and characterisation in affected brains one of the major challenges in amyloid research.
Synchrotron-based microscopy techniques can help elucidate the structure and composition of amyloid plaques. Infrared microscopy is based on the absorption of infrared light, which gives information about the chemical composition of samples. In biological samples, it is especially interesting to be able to measure parameters such as protein/peptide secondary structure and lipid oxidation. The technique is particularly useful in the case of amyloid aggregation, since the aggregated peptides/proteins generate very specific and easily identifiable spectroscopic bands in the mid-infrared regions (between 1620 and 1630 cm-1) [2].
A team of researchers from Barcelona in collaboration with scientists from the ESRF have used infrared microscopy at beamline ID21 and at the ALBA synchrotron to characterise the amyloid aggregates in brain samples from patients in advanced stages of Alzheimer’s disease. Two different types of amyloid peptide aggregates were found: fibrils (which have been previously described) and – identified for the first time in affected brains – non-fibrillary aggregates with a high component of disordered structure. Moreover, the spectroscopic analysis allowed the identification of oxidised lipid components associated with the amyloid plaques.
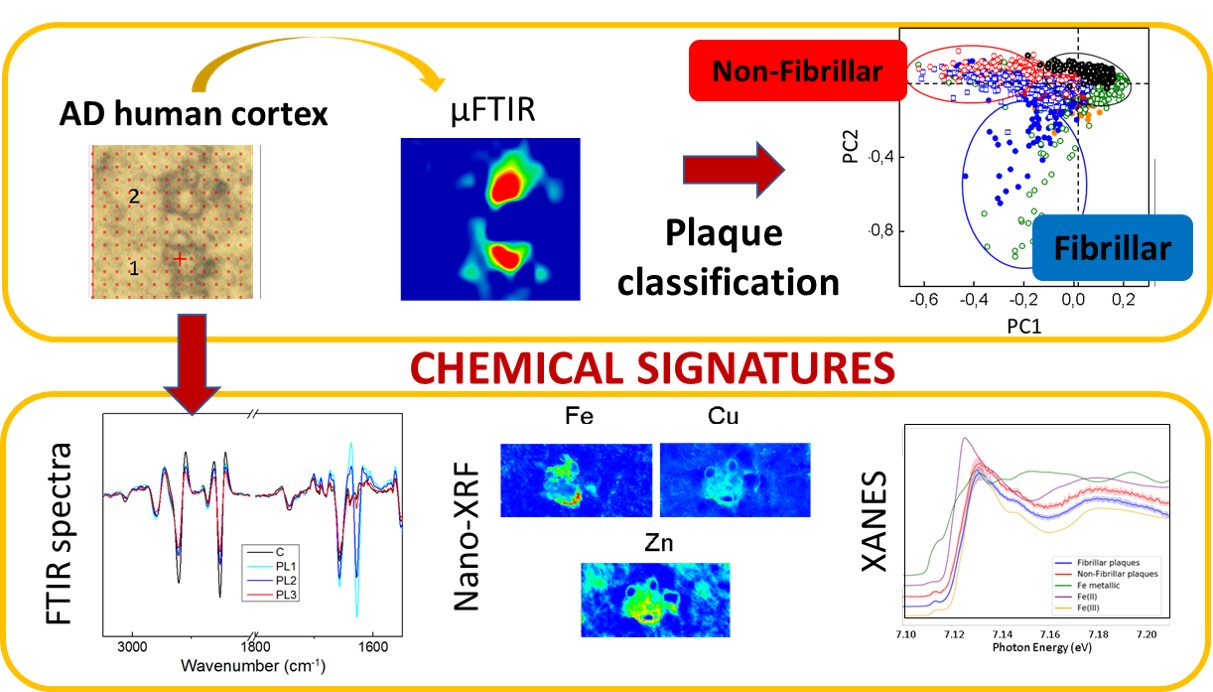
Fig. 1: Scheme of the experiments carried out. Amyloid plaques were first classified between fibrillary and non-fibrillary using infrared (IR) microscopy. After the characterisation, the metal composition and the iron oxidation state were studied using X-ray fluorescence (XRF) and X-ray absorption near-edge spectroscopy (XANES).
The role of metal cations is also a highly discussed topic in relation to Alzheimer’s disease since metals, together with the amyloid peptides, may be involved in a redox reaction that could lead to oxidative stress, which could be related to the development of the disease. Combining infrared microscopy with X-ray fluorescence (XRF) measured at beamline ID16A allowed the researchers to study the correlation between different types of amyloid deposits and their metal ion composition (Figure 1). In agreement with previous studies, the concentration of metals was found to be higher within the amyloid plaques than in the surrounding area. The nano-XRF distribution maps show that zinc and copper clearly co-localise with the amyloid deposits, while iron appears to be associated with the plaque border (Figure 2).
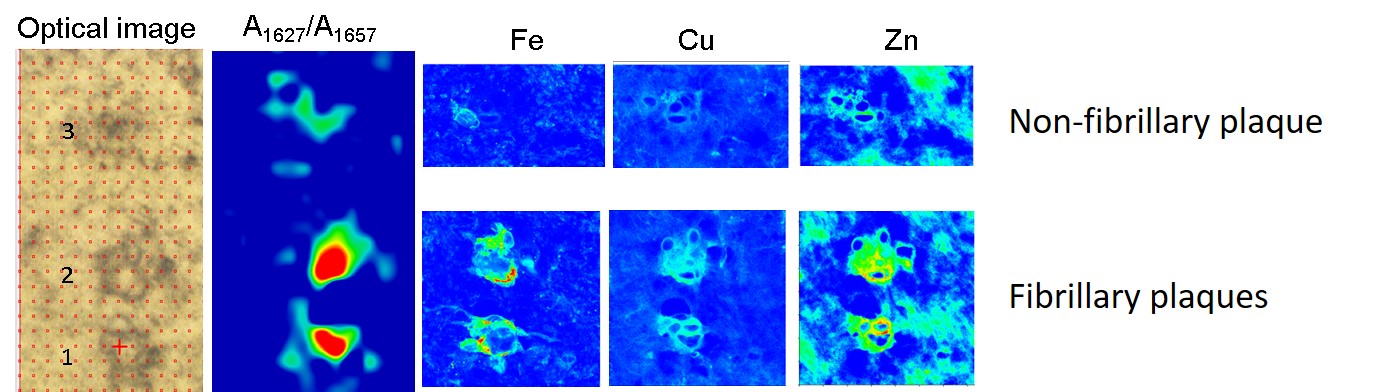
Fig. 2: Example of the metal distribution in fibrillary and non-fibrillary plaques.
As for the different types of amyloid plaque, the concentration of iron was found to be higher in fibrillary plaques than in non-fibrillary ones, whereas the levels of copper and zinc were found to be similar in both types of amyloid deposits. X-ray absorption near-edge spectroscopy (XANES) measurements carried out at beamline ID21 show that the non-fibrillary amyloid plaques (with increased disordered structure) contain both Fe2+ and Fe3+, whereas fibrillary plaques contain mainly Fe3+.
In conclusion, the existence of amyloid deposits with disordered/non-fibrillar structure, coexisting with the typical fibrillary plaques, has been described for the first time in situ in human brains with advanced Alzheimer’s disease. Both disordered and fibrillary plaques accumulate metal ions, although the iron content is clearly increased in the fibrillary deposits. Non-fibrillary plaques contain Fe2+ (not present in the fibrillary plaques) and are associated with higher levels of lipid oxidation. The presence of redox-active form Fe2+ in non-fibrillary plaques may be key for its participation in the redox processes leading to increased oxidative stress.
Identification, localisation, and characterisation of potentially toxic non-fibrillary amyloid species in the affected brains may be of relevance in Alzheimer’s disease research since these forms may precede the formation of (non-toxic) fibrils, and be more directly related to the harmful effects of the amyloid peptide in the development of the pathology. Future experiments to study the presence of these non-fibrillar forms at early stages of the disease and its metal cation content would be of great interest for the research field.
Principal publication and authors
Synchrotron X-ray Fluorescence and FTIR Signatures for Amyloid Fibrillary and Nonfibrillary Plaques, E. Álvarez-Marimon (a), H. Castillo-Michel (b), J. Reyes-Herrera (b), J. Seira (a), E. Aso (c), M. Carmona (c), I. Ferrer (c), J. Cladera (a), N. Benseny-Cases (d), ACS Chem. Neurosci. 12(11), 1961-1971 (2021); https://doi.org/10.1021/acschemneuro.1c00048.
(a) Unitat de Biofísica, Universitat Autonoma de Barcelona, Catalonia (Spain)
(b) ESRF
(c) Institut de Neuropatologia, IDIBELL-Hospital Universitari de Bellvitge, Universitat de Barcelona (Spain)
(d) ALBA Synchrotron Light Source, Catalonia (Spain)
References
[1] K.L. Stewart et al., Biophys. Rev. 4, 405-419 (2017).
[2] N. Benseny-Cases et al., Anal. Chem. 86, 24, 12047-12054 (2014).



