- Home
- News
- Spotlight on Science
- Molecular layering...
Molecular layering of ionic liquids
06-01-2009
Room-temperature ionic liquids are promising candidates for a broad range of "green" applications, for which their interaction with solid surfaces plays a crucial role. In this study, the temperature-dependent structures of three ionic liquids in contact with a charged sapphire substrate were investigated with sub-molecular resolution. Strong molecular layering was observed with decay length and layering period pointing to an interfacial ordering mechanism originating from strong correlations between the unscreened ions.
Room temperature ionic liquids (RTILs) are comprised of molecular ions. They are interesting materials from a physical point of view as they allow us to modify the interactions between the molecular ions as well the interaction with other materials by simply modifying the individual ionic molecules chemically [1]. Up to now several thousand ionic liquids have been synthesised and many of them are available from commercial sources.
Room temperature ionic liquids are also very promising candidates for a variety of new technological applications, such as solvents in green chemistry, catalysis, batteries, and fuel and solar cells. Although crucial for the understanding of their properties in applications, little is known about the structural arrangement of the anions and cations at solid interfaces. To get access to these interfaces, high energy X-ray reflectivity (E = 72.5 keV) at the instrument HEMD for high-energy microdiffraction at beamline ID15A has been employed. This is an ideal tool for penetrating to deeply buried interfaces within bulk samples. The X-ray reflectivity was measured and could then be used to extract information on the laterally-averaged electron-density profile at the RTIL/Al2O3 interface.
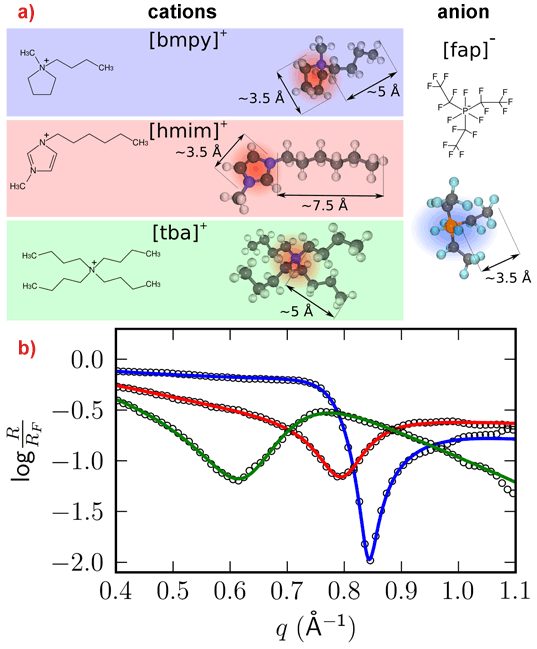
Systematic studies of the interfacial structure as a function of composition and temperature were performed with several RTILs at an Al2O3(0001) surface. By choosing different combinations of anions and cations, it was possible to tune the ion-ion and ion-substrate interaction. By changing the temperature, the ratio between entropy and interfacial energy could be varied, driving the system closer to a disordered liquid or interfacial ordering, respectively. Figure 1 shows the normalised high-energy reflectivity for three different RTILs containing the same anion [fap]-. All three measurements show distinct features in the normalised reflectivity that could be attributed to pronounced molecular layering at the RTIL/Al2O3 interface. The measurements are perfectly reproduced by a modified distorted crystal model. Figure 2 shows an example, the reconstructed density of the interface [fap]-[bmpy]+/Al2O3(0001). The first layer at the interface is comprised of cations, which appears counterintuitive at first sight, followed by a second layer of anions, thus ensuring charge neutrality. Additional measurements employing a Kelvin probe confirmed that the substrate is negatively charged, thereby explaining the formation of a purely cationic layer directly at the interface. The molecular layering decays exponentially with a decay length of approximately two molecular double layers. Similar results have been found for the other two interfaces [fap]-[hmim]+/Al2O3 and [fap]-[tba]+/Al2O3.
Dilute aqueous electrolytes containing water-screened weakly-interacting ions have been successfully described by mean field theories of charge double layers. This description neglects, however, correlations between ions. In contrast, the interaction between the oppositely-charged RTIL ions is very strong because no other molecule is available for screening. The ions are, therefore, strongly correlated. Such correlations lead to other apparently counterintuitive phenomena like charge inversion and attraction between like-charged objects. Our findings are corroborated by recent molecular dynamics simulations of (simpler) RTILs in slit pores with Lennard-Jones potentials for the dispersion interactions of the molecules as well as for the wall-molecule interaction, where a clear separation of the anions and cations into distinct layers was also observed [2]. Information on the structure of such interfaces should help us to develop a deeper understanding of their properties such as their transport properties, useful for applications.
References
[1] P. Wasserscheid, T. Welton, Ionic liquids in synthesis (Wiley-VCH, Weinheim Germany, 2007).
[2] C. Pinilla, et al., J. Phys. Chem. B 111, 4877 (2007).
Principal publication and authors
M. Mezger (a), H. Schröder (a), H. Reichert (a), S. Schramm (a), J.S. Okasinski (a), S. Schröder (a,b), V. Honkimäki (b), M. Deutsch (c), B.M. Ocko (d), J. Ralston (e), M. Rohwerder (f), M. Stratmann (f), H. Dosch (a,g), Science 322, 424 (2008).
(a) Max-Planck-Institut für Metallforschung, Stuttgart (Germany)
(b) ESRF
(c) Physics Department, Bar-Ilan University, Ramat-Gan (Israel)
(d) Condensed Matter Physics and Materials Science Department, Brookhaven National Laboratory, Upton (USA)
(e) Ian Wark Research Institute, University of South Australia, Mawson Lakes (Australia)
(f) Max-Planck-Institut für Eisenforschung, Düsseldorf (Germany)
(g) Institut für Theoretische und Angewandte Physik, Universität Stuttgart (Germany)
![Electron density profile of the interface [fap]-[bmpy]+/Al2O3 at T=-15°C.](/files/live/sites/www/files/news/spotlight/spotlight74/spotlight74_fig2sm.gif)



