- Home
- News
- General News
- ESRF helps unpack...
ESRF helps unpack mechanism of DNA repair
01-12-2023
Many organisms use the photo-enzyme “photolyase” to repair sun-damaged DNA. Now, a team at the ESRF’s icOS Lab has analysed time-resolved data from free-electron lasers to crack open its repair mechanism. Published today in Science, the study paves the way for enhanced time-resolved imaging at the ESRF’s ID29 beamline.
The sun’s ultraviolet light is highly damaging to DNA. It triggers the formation of extra covalent bonds within the same DNA strand, preventing cells from reading the DNA-encoded gene. In humans and many other mammals, a molecular process known as nucleotide excision performs the repair, but it is not very efficient. Much more efficient and simpler is the function of photolyase, which is employed by plants, bacteria, fungi and all animals – except those with placentas, such as humans.
Potentially, a greater understanding of how photolyase works could lead to the development of drugs to treat DNA damage – caused by the sun, or ageing – in humans. But a more immediate lure for scientists is its rarity: it is one of just three known photo-enzymes – that is, proteins that catalyse chemical reactions by harnessing light. Back in 2017, the ESRF was involved in the discovery of one of the others, fatty acid photodecarboxylase, which synthesises hydrocarbons in microalgae.
In this study, a team of scientists led by the Academica Sinica and the National Taiwan University in Taipei, Taiwan, including colleagues at Osaka University in Japan, Philipps University Marburg in Germany and the ESRF, have investigated the mechanism of photolyase using data from two X-ray free-electron lasers (XFELs): SACLA in Japan’s SPring-8 accelerator complex and SwissFEL at the Paul Scherrer Institute in Switzerland. At the ESRF, the in crystallo Optical Spectroscopy (icOS) laboratory, headed by Antoine Royant, verified that the crystals were in the necessary reduced state. “It’s a magical process to be able to repair DNA with a protein and just a flash of light,” says Royant. “We just wanted to know how nature managed to solve such a complicated problem.”
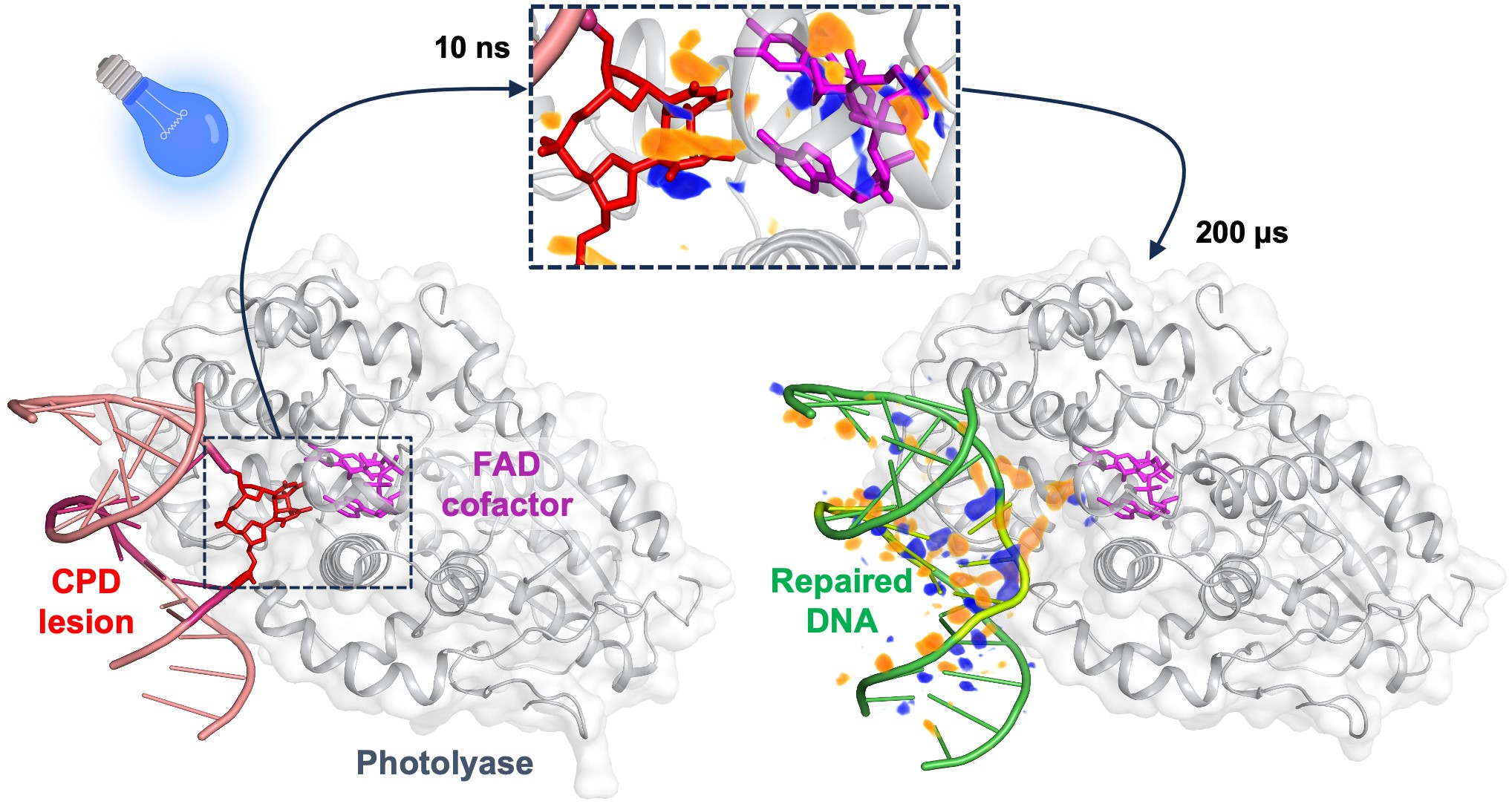 |
|
On the left, the damaged bases of a DNA strand point towards the interior of the photolyase enzyme. Upon absorption of blue light by the FAD cofactor, the covalent bonds of the damaged DNA are ruptured within 10 nanoseconds, as illustrated by the difference electron-density map (upper insert, with difference electron density in orange and blue). After 200 microseconds, the repaired bases reorient towards the interior of the double-strand DNA, which is then ready to dissociate from the enzyme. Credits: Maestre-Reyna, M. et al. Science, 1 December 2023. |
The crucial ESRF contribution, however, was not in crystal verification but in data analysis. For every XFEL snapshot over a time range of 100 picoseconds to 200 microseconds, the crystallographic data included contributions from several intermediate species of photolyase in varying proportions. A conventional, restricted analysis of the data would have given ambiguous results. Together with ESRF PhD student Nicolas Caramello, therefore, Royant provided an unambiguous, global analysis using a specialist computational technique known as singular value decomposition (SVD), which encodes the raw data of any single snapshot within a huge matrix. Based on linear algebra, SVD is able to break down this matrix into a series of three-dimensional mathematical objects, which are representative of the main forces that evolve during the reaction. “It involves manipulating big arrays with lots of numbers – gigantic vectors,” says Royant. “You don’t take this tool off the shelf.”
The SVD analysis showed that the photolyase mechanism proceeds in a series of clear, sequential steps. In the beginning, following the absorption of a photon of visible light by the photo-enzyme, electrons from part of the photolyase transfer to the DNA. In the next step, the two extra covalent bonds, formed by ultraviolet damage, are broken one after the other, before the different chemical groups involved in the reaction rearrange. Finally, the two bases of the repaired DNA strand turn from the side of the photo-enzyme back inside the DNA helix, preparing the DNA to break away. “What we’ve created is a true molecular film of the events, from the absorption of a light photon to the complete repair of the DNA,” says Royant.
The results provide an unprecedented insight into the mechanism of a rare photo-enzyme. But, according to Royant, they also demonstrate the power of SVD analysis for time-resolved crystallography. The ESRF’s flagship beamline ID29 is one of the world’s first synchrotron beamlines dedicated to serial crystallography, and benefits from the EBS flux to study molecular mechanisms at microsecond time resolution. “SVD will be a tool for ESRF users as well,” says Royant.
Reference:
Maestre-Reyna, M. et al, SCIENCE, 1 Dec 2023, Vol 382, Issue 6674. DOI: 10.1126/science.add7795
Text by Jon Cartwright



