- Home
- News
- Spotlight on Science
- Multiscale X-ray...
Multiscale X-ray techniques disclose the active phase of bismuth electrocatalysts
06-11-2023
Combined in-situ X-ray absorption spectroscopy and X-ray diffraction at beamline ID26 have disclosed the activation of bismuth oxyhalide nanoparticles during the electrocatalytic conversion of CO2 into formate. The results could help in the rational design of electrocatalysts for renewable energy.
The electrocatalytic conversion of carbon dioxide (CO2) into valuable base chemicals depends highly on the catalyst structure and composition. Changes in the catalyst structure, such as morphology, exposed surface facets, and composition, all have a drastic influence on the electrocatalytic performance. A variety of electrocatalytic materials have been investigated, but the actual active phase under reaction conditions remains heavily debated in the literature. For example, post-transition metals, such as Pb, Bi, Sn, and In, show promise for selective CO2 conversion to formate, but the exact structure of the active electrocatalyst remains largely unknown. Exploring the catalyst structure and composition under realistic reaction conditions, over multiple length scales with high spatiotemporal resolution, is thus crucial. X-ray radiation is ideal for probing the catalyst structure and composition under realistic reaction conditions, penetrating the aqueous electrolyte without damage.
Combining in-situ X-ray diffraction (XRD) and X-ray absorption spectroscopy (XAS) at beamline ID26, this work reveals the active phase of bismuth electrocatalysts during CO2 reduction. The results show that the presence of bromide in the pristine bismuth oxyhalide nanomaterials drives the formation of planar bismuth surfaces, which display high selectivity to formate (>90%) at high current density (150 mA/cm2). An investigation into the effect of halides on the activation of the bismuth-based electrocatalysts revealed that chloride ions promote stepped Bi(012) surfaces, whereas iodide-activated electrocatalysts consist of both planar and stepped surfaces, suggesting a direct relationship between halide-activation, active phase and catalytic performance.
Click image to enlarge
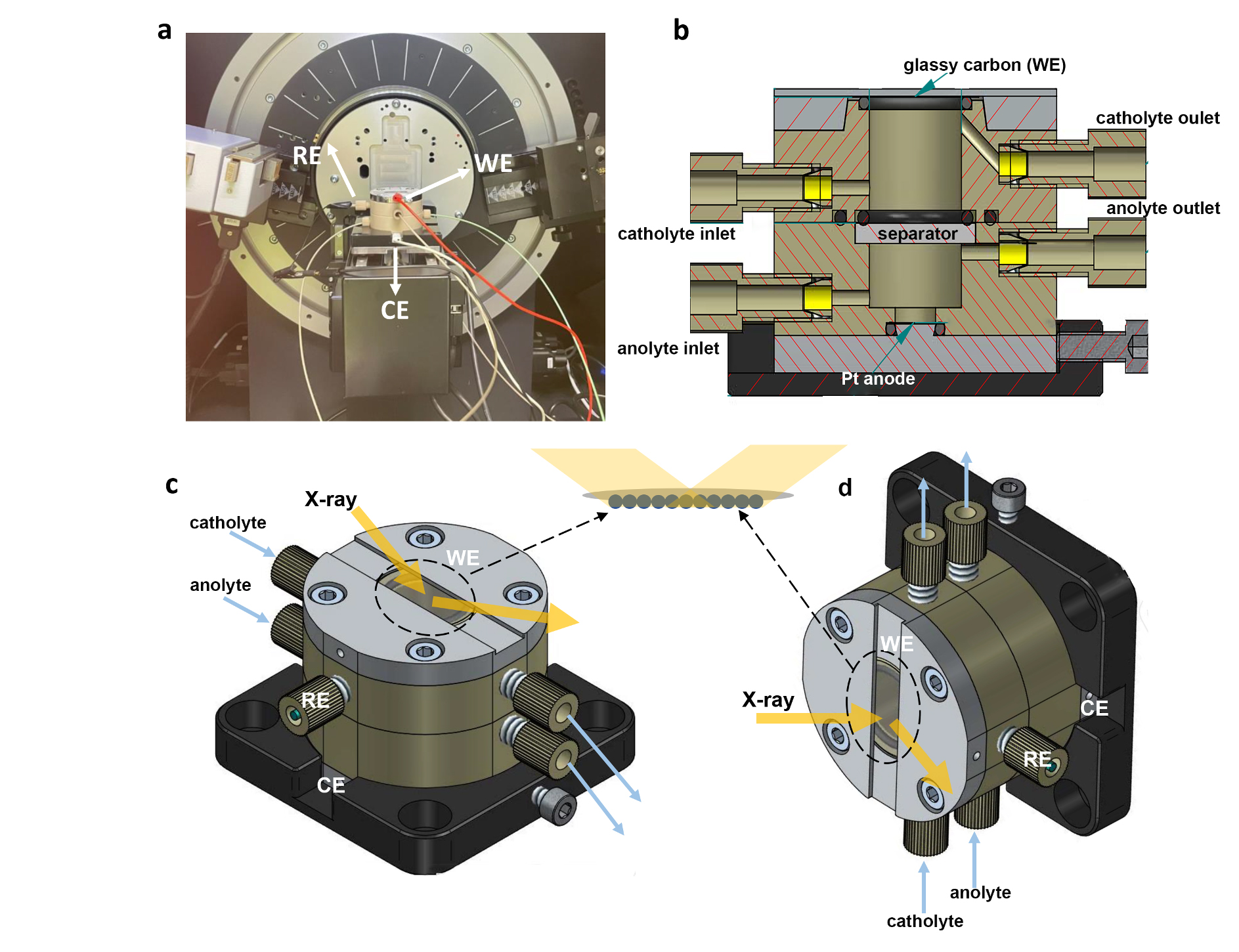
Fig. 1: a) Picture of the in-situ XRD setup and (b) internal schematic of the in-situ X-ray electrochemical cell. c) External schematic of the in-situ XRD cell configuration and (d) in-situ XAS cell configuration. The working electrode (WE), counter electrode (CE) and reference electrode (RE) are located at the top, bottom, and side position of the cell, respectively. The separator can be an ion-exchange membrane or a quartz filter. The WE is a round glassy carbon wafer with a thickness of 180 µm and a diameter of 20 mm, covered by a catalyst layer. Back illumination mode was used for all the in-situ XRD and XAS measurements.
One of the key components for successful in-situ investigations of electrocatalysts at work is the cell design. In this case, an in-house cell design was used, in which the electrocatalyst is probed in back-illumination mode (Figure 1) to circumvent possible scattering from the aqueous electrolyte. The study began by synthesising a series of layered bismuth oxyhalide nanoplatelets (BiOX, with X = Cl, Br, and I) to serve as templates for well-defined bismuth electrocatalysts. Characteristic XRD (001) reflections and their overtones confirmed the layered nature of these templates, which exhibited good catalytic properties upon in-situ activation during electrocatalytic CO2 reduction (Figure 2a). Intriguingly, the presence of various halides in the pristine electrocatalyst seemed to dominate the catalytic performance: bromide-activated electrocatalysts clearly outperformed the chloride- and iodide-activated electrocatalysts, with efficiencies exceeding 90% at high current density of 150 mA/cm2.
Click image to enlarge
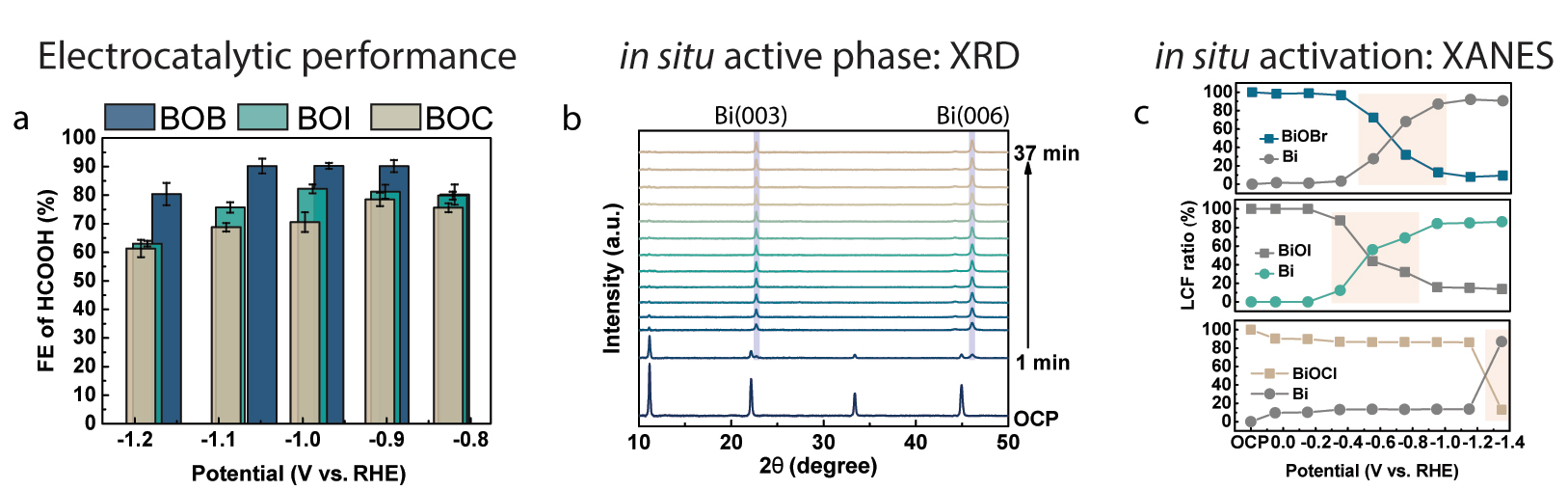
Fig. 2: a) Comparison of potential-dependent Faradaic Efficiency (FE) of formate for bismuth oxybromide (BOB), -chloride (BOC) and -iodide (BOI). b) Time-dependent in-situ XRD patterns at a fixed potential of −1.15 V vs. RHE for BOB, showing selective exposure of planar Bi surfaces. c) The Linear Combination Fitting (LCF) results of the potential-dependent in-situ X-ray absorption near-edge structure (XANES) spectra for BOB, BOI and BOC, revealing the halide-guided variations in activation toward metallic bismuth.
Multiscale X-ray characterisation was then used to investigate the role of halides in the activation of Bi electrocatalysts, and to determine the electrocatalytic CO2 reduction reaction mechanism. Potential- and time-dependent in-situ XRD revealed the halide-guided exposure of planar facets (in the case of bromide) and stepped surfaces (in the case of chloride and iodide) (Figure 2b). Meanwhile, it was observed that a higher overpotential was required to activate the chloride-containing nanoparticles than the bromide and iodide counterparts. To investigate the role of the halides in the active structure in more detail, in-situ XAS at the Bi L3-edge were conducted (Figure 2c). These measurements, along with subsequent linear combination fitting to the reference compounds, revealed complete conversion of the bismuth oxyhalide templates into metallic bismuth. Furthermore, the activation barrier for the chloride-containing templates was much higher than that of the bromide and iodide counterparts, which is in line with the in-situ XRD results. These observations suggest that planar bismuth surfaces are more selective for electrocatalytic CO2 conversion into formate and that halides can be used to direct the in-situ activation of the electrocatalysts.
This work provides multiscale X-ray insights into the active phase of in-situ activated Bi-based electrocatalysts during CO2 reduction into formate, potentially paving the way for the rational design of other electrocatalyst materials for renewable production of chemicals and fuels.
Principal publication and authors
Halide–guided active site exposure in bismuth electrocatalysts for selective CO2 conversion to formate, S. Yang (a), H. An (a), S. Arnouts (b,c), H. Wang (a), X. Yu (a), J. de Ruiter (a), S. Bals (b), T. Altantzis (c), B.M. Weckhuysen (a), W. van der Stam (a), Nat. Catal. 6, 796-806 (2023); https://doi.org/10.1038/s41929-023-01008-0
(a) Inorganic Chemistry and Catalysis, Debye Institute for Nanomaterials Science and Institute for Sustainable and Circular Chemistry, Utrecht University (The Netherlands)
(b) Electron Microscopy for Materials Science (EMAT), University of Antwerp (Belgium)
(c) Applied Electrochemistry and Catalysis (ELCAT), University of Antwerp (Belgium)
| About the beamline: ID26 |
|
ID26 is dedicated to X-ray absorption and emission spectroscopy of complex systems in the tender and hard X-ray range. The high-brilliance X-ray beam allows for spectroscopic studies of samples with low analyte concentration and challenging matrix. X-ray emission spectroscopy is performed by means of crystal analyzer spectrometers. By combining the tuneable incident energy with an emission spectrometer, it is possible to take advantage of resonance effects that can provide detailed information on the electronic structure. The local coordination and electronic structure of an X-ray absorbing atom are studied by extended X-ray absorption fine structure (EXAFS), X-ray absorption near edge structure (XANES), X-ray emission (XE) and resonant inelastic X-ray scattering (RIXS) spectroscopy. The techniques probe occupied and unoccupied electron orbitals, providing a wealth of information. It is thus possible to study orbital splittings, spin- and oxidation states as well as the coordination symmetry and ligand type. RIXS gives access to element-specific excitations of only a few eV that can arise from local (e.g., d-d), nearest neighbour (e.g., charge transfer) and collective excitations. With the tender and hard X-ray probe, very few restrictions apply to the sample environment. ID26 can host cryostats and cells for in-situ and operando studies to carry out experiments in applied sciences including coordination chemistry, (bio)catalysis, materials science, electro-chemistry and environmental sciences. |