- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Complex systems and biomedical sciences
- Element-specific depth profiles in biomolecular layers
Element-specific depth profiles in biomolecular layers
Molecular layers are major components of all biological systems and they are very important for biotechnological applications. The understanding of biological processes in molecular layers requires detailed structural insight. Standing-wave X-ray fluorescence (SWXF) is a label-free and element-specific technique for the structural investigations of molecular layers, at atom-scale resolution, perpendicular to the interface. It is therefore a powerful tool for the structural analysis of biological and biotechnologic surfaces.
In nature biomolecules are often organised as functional thin layers in interfacial geometries. The most prominent examples are biological membranes. But biomolecular layers also play important roles at biotechnological surfaces, for example, when they are the result of adsorption processes. For the understanding of many biological or biotechnologically relevant processes, a detailed insight into the structure of such layers is crucial.
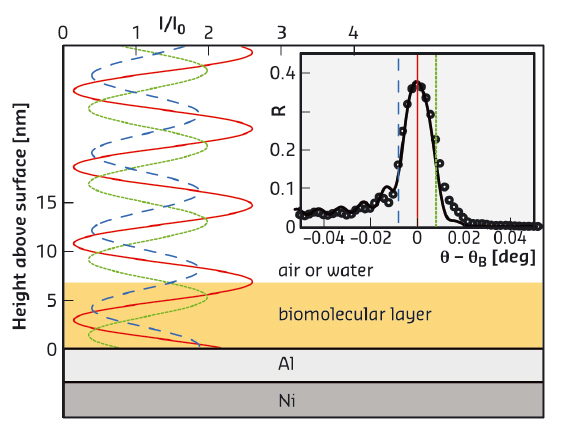
X-ray and neutron scattering are particularly well suited for the investigation of such layers. In contrast to commonly used specular reflectometry, which reveals “global” matter density profiles perpendicular to a planar interface, standing-wave X-ray fluorescence (SWXF) allows the density profiles specifically of chemical elements of interest to be determined. SWXF is based on the standing wave created above a solid surface by the interference of the incident X-ray wave with the wave reflected from a multilayered solid substrate around the Bragg condition. During a scan of the incidence angle θ across the Bragg peak at θB, the maxima of the standing wave move along the surface normal (z, see Figure 106) and induce X-ray fluorescence with element-characteristic energies.
The angle-dependent fluorescence intensity Ij(θ) of a target element j is proportional to the overlap of its density profile perpendicular to the interface, ρφ(z), and the angle-dependent standing wave Φ(θ, z),
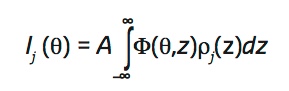
 |
|
Fig. 106: X-ray standing wave above the solid surface for various incident angles θ around the Ni/Al multilayer Bragg angle θB. The characteristic fluorescence of the target is enhanced when the maxima of the standing wave overlap with the element distribution. (Inset) X-ray reflectivity (symbols) around θB and modelled reflectivity curve (solid line). Vertical lines indicate the incident angles corresponding to the standing waves in the main panel. |
The method thus allows elemental depth profiles to be reconstructed from the angle-dependent fluorescence [1, 2]. In fact, the centre of mass position of an elemental distribution can be determined with atom-scale resolution. SWXF studies have so far dealt with relatively heavy elements, typically metal ions, as artificial labels for the molecular layers under investigation [2].
In recent experiments at beamline ID03 and at SOLEIL/DIFFABS employing specially-designed Ni/Al multilayer substrates, we have demonstrated that the SWXF approach can be extended also to light, biologically-relevant chemical elements such as sulfur and phosphorus, which are found in the most important classes of biomolecules and therefore particularly interesting.
 |
|
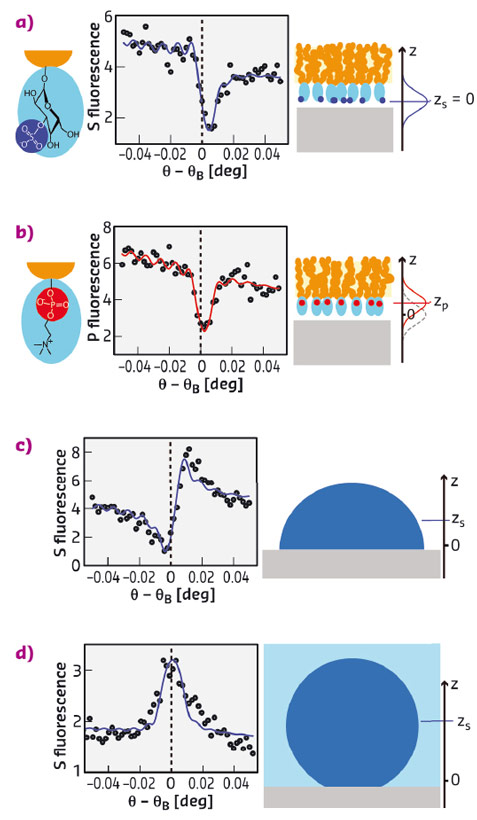
Fig. 107: Experimental results on sulfoglycolipid (a) and phospholipid (b) monolayers in air as well as on protein monolayers in air (c) and under water (d), all on Al oxide surfaces. The graphs show the angle-dependent P and S fluorescence intensities (symbols) together with the simulated intensities (solid lines) corresponding to the best-matching models. Vertical dashed lines indicate the Bragg condition. Cartoons on the right illustrate the architectures of the layers studied. The axis perpendicular to the sample surface is denoted z. |
Figure 107 summarises experimental results obtained with sulfoglycolipid, phospholipid, and human serum albumin (HSA) monolayers on Al oxide surfaces. Phospholipids and sulfoglycolipids are abundant lipid classes in eukaryotic cell membranes that contain P and S atoms, respectively, in their headgroups (see Figure 107a,b). The protein human serum albumin (HSA) is the most abundant protein in the human blood serum and carries numerous S atoms in the cysteine and methionine amino acids distributed throughout its molecular structure. The graphs in Figure 107a-d show angle-dependent S and P fluorescence intensities (symbols) together with the simulated intensities (solid lines) corresponding to the best-matching elemental distributions. Distinct differences between the angle-dependence of the S and P fluorescence intensities from sulfoglycolipid (Figure 107a) and phospholipid (Figure 107b) monolayers, respectively, revealed an approximately 4 Å height difference of the sulfate and phosphate groups with respect to the solid surface. Dramatic differences are observed when comparing the shape of the fluorescence curves in (Figure 107a,b) with those of the S fluorescence intensities obtained with HSA for various conditions (Figure 107c,d). The differences in the angle dependence reflect that the S distribution of the adsorbed HSA monolayers is centred much higher above the surface, at approximately 17 Å in air and 37 Å under water.
These results demonstrate that our label-free implementation of SWXF allows the investigation of complex biomolecular interfaces with great structural detail.
Principal publication and authors
Atom-scale depth localization of biologically important chemical elements in molecular layers, E. Schneck (a,b), E. Scoppola (b,c), J. Drnec (d), C. Mocuta (e), R. Felici (d), D. Novikov (f), G. Fragneto (b) and J. Daillant (e), PNAS 113, 9521-9526 (2016); doi: 10.1073/pnas.1603898113.
(a) Max Planck Institute of Colloids and Interfaces, Potsdam (Germany)
(b) Institut Laue-Langevin, Grenoble (France)
(c) Institut de Chimie Séparative de Marcoule, Bagnols sur Cèze (France)
(d) ESRF
(e) Synchrotron SOLEIL, Gif-sur-Yvette Cedex (France)
(f) Deutsches Elektronen-Synchrotron DESY, Hamburg (Germany)
References
[1] E. Schneck et al., PNAS 107, 9147 (2010).
[2] E. Schneck and B. Demé, Curr. Opin. Colloid Interface Sci. 20, 244 (2015).



