- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Enabling technologies
- Direct atomic force microscopy imaging at liquid interfaces
Direct atomic force microscopy imaging at liquid interfaces
In this study we show that atomic force microscopy (AFM) can be used to image films at liquid/liquid interfaces providing local and quantitative structural information.
The assembly of nanoscopic objects and molecules at liquid interfaces is currently attracting extensive research interest as it can be employed to prepare functional monolayers and membranes for relevant technological applications [1]. The precise control of the assembly process requires a thorough structural characterisation which, so far, has mainly been carried out by reciprocal space techniques, namely X-ray and neutron scattering and reflectivity [2]. While these methods provide invaluable high-resolution and statistically significant data, the information obtained is averaged over macroscopic areas, preventing detailed characterisation of individual nanoscale defects and direct observation and manipulation of localised nanostructures. This characterisation can be performed only by microscopies but, in the case of liquid interfaces, it has so far been limited to molecules with specific functionalities.
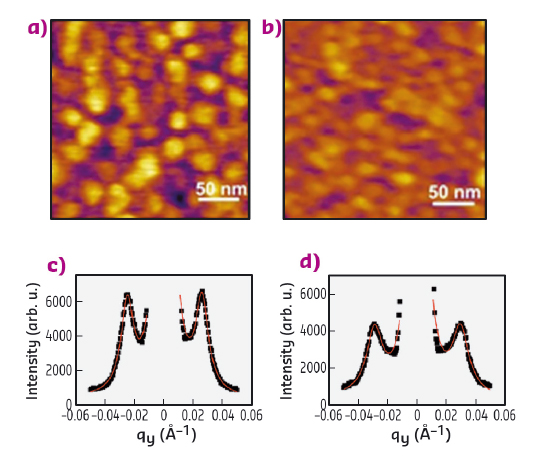
In this study, we show that atomic force microscopy (AFM) can be applied to image films at liquid/liquid interfaces providing local and quantitative structural information by taking into account certain experimental precautions. AFM has been used for decades to characterise solid and molecular films supported on solid substrates with nanometric resolution and without the need for any specific treatment. We have proven AFM’s applicability also to liquid-liquid interfaces by imaging cetyltrimethylammonium bromide (CTAB)-decorated silica nanoparticle monolayers at the water/heptane interface and comparing the results with independent grazing-incidence small-angle X-ray scattering (GISAXS) measurements performed at beamline ID10. Indeed, by varying the CTAB concentration, it is possible to tune the spacing between adjacent interfacially adsorbed nanoparticles with nanoscale precision. In Figure 158 the topographical images of silica nanoparticle monolayers in the presence of 0.01 mM (a) and 0.05 mM (b) CTAB are reported. These images were acquired with a Cypher AFM (Asylum Research, Oxford Instruments) that is now available at the AFM platform of the Partnership for Soft Condensed Matter (PSCM). The average centre-to-centre distance between adjacent nanoparticles was determined to be 28.7 ± 2.7 and 24.6 ± 2.5 nm respectively by counting the number of nanoparticles in each image and with the assumption of hexagonal packing. These values are consistent with those determined from the GISAXS measurements reported in Figure 158c-d, 28.4 ± 0.6 and 24.7 ± 0.3 nm respectively.
 |
|
Fig. 158: AFM topographical images of nanoparticle monolayers with CTAB 0.01 mM (a) and 0.05 mM (b), GISAXS 1D profile of the nanoparticle monolayers with CTAB 0.01 mM (c) and 0.05 mM (d). |
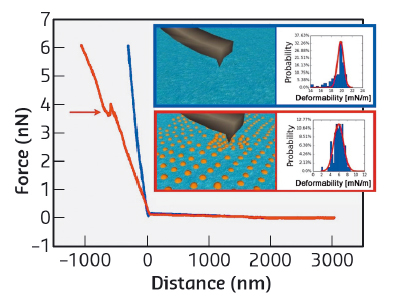
In addition to topographical data, AFM can also measure force-distance curves providing information about the interaction force between the AFM probe and the liquid interface. Two typical force-distance curves are reported in Figure 159. Once the contact between the AFM probe and the interface is established, that is at distance 0, the probe starts indenting the interface over several hundreds of nanometres (distance < 0). Interestingly, the liquid interface without nanoparticles (blue curve) is stiffer. As the deformation of the interface is resisted by its interfacial tension, the lowering of the slope in the presence of surfactant-nanoparticle complexes can be interpreted in terms of a reduction of the interfacial tension. Thus, with suitable modelling, force distance curves at the liquid interfaces can be employed to locally measure the interface tension with nanoscale resolution, providing a significant improvement with respect to current macroscale tensiometry. Finally, the force curve in the presence of nanoparticles shows a jump, highlighted by the arrow. This represents the maximum force an interfacial nanoparticle can withstand before being displaced by the nanoscale apex of the AFM tip. Such force information could potentially be used to quantify local in–plane interfacial interactions between individual nanoparticles.
 |
|
Fig. 159: Typical force-distance curves of the water/heptane interface in the absence (blue) and in the presence (red) of a silica nanoparticle monolayer. The insets show the distribution of the linear slope (deformability) obtained from several independent curves. |
In conclusion, this study opens the way to a new investigation approach for liquid-liquid interfaces. AFM imaging can be applied to any kind of molecule or object capable of assembling at liquid interfaces with high coverage once the system is at equilibrium. The results obtained are not limited to structure, as local information on interfacial tension and interfacial forces can be obtained. These findings will help to control and optimise the structure of monolayers and films at liquid interfaces and to characterise more complex systems, such as biological membranes, where the identification of domains with different assembly and properties is necessary for the full comprehension of several biophysical processes.
Principal publication and authors
L. Costa (a,c), G. Li Destri (a), N.H. Thomson (b), O. Konovalov (a) and D. Pontoni (a), Nano Letters 16, 5463-5468 (2016); doi: 10.1021/acs.nanolett.6b01877.
(a) ESRF
(b) School of Physics and Astronomy, University of Leeds (UK)
(c) Present address: Centre de Biochimie Structurale, Montpellier (France)
References
[1] L. Hu et al., Chem. Soc. Rev. 41, 1350-1362 (2012).
[2] L. Cristofolini, Curr. Opin. Colloid Interface Sci. 19, 228-241 (2014).



