- Home
- News
- Spotlight on Science
- X-ray techniques...
X-ray techniques lend new insights into how the liver processes silver nanoparticles
04-09-2023
X-ray fluorescence nano-imaging and X-ray absorption spectroscopy were used at beamlines ID16B and BM30 to investigate how a cellular model that mimics the liver metabolises silver nanoparticles. The results unveil the molecular mechanisms linked to the human toxicity of a broadly used nanomaterial.
The growing use of nanotechnologies for their innovative material properties has led to concerns about their potential toxicity to humans. Silver nanoparticles (AgNPs), which are widely used for their antibacterial properties in medical devices (e.g., catheters and burn dressings) and consumer products (e.g., food packaging), are one example of a nanomaterial with potential toxicity. Humans can therefore be exposed to AgNP parenterally (e.g., through intravenous injection), where intact AgNP enter the bloodstream, or orally, where AgNP are transformed into Ag(I) ions in the gastro-intestinal tract; in both cases, silver accumulates in the liver. Unravelling the fate of AgNP in the liver and the excretion mechanisms in these different exposure conditions is thus essential to evaluate the toxicity of AgNP and define a regulatory framework for their safe use. The effects of AgNP on mammals have been studied using both cellular and animal models. However, while the former reveal molecular mechanisms occurring at the subcellular level, and the latter bring insight into toxicity and accumulation at the organ level, it has not yet been possible to reconcile these two kinds of studies in a translational approach.
In this work, an innovative 3D cellular model that partially reproduces the liver architecture and metabolism was used in exposure scenarios mimicking parenteral and oral exposure to AgNP, helping to bridge the gap between cell cultures and animal models. The cellular model consisted of 3D cultures of HepG2/C3A hepatocytes, which form spheroids of interconnected cells and functional bile canaliculi (i.e., the tubular structures where excretion takes place). The spheroids were exposed to two commonly used AgNP, coated with citrate (cit) or polyvinylpyrrolidone (PVP), or to a silver salt (AgNO3), in order to mimic parenteral and oral exposure, respectively. X-ray fluorescence (XRF) nano-imaging, X-ray absorption spectroscopy (XAS) and electron microscopy were used to track Ag species in the liver, from uptake to transformation and excretion.
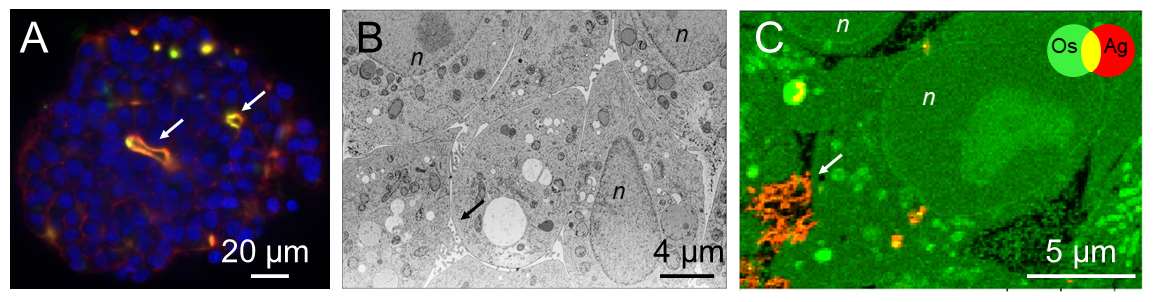
Fig. 1: A) Light sheet fluorescence microscopy image of a hepatocyte spheroid stained for actin (red), cell nuclei (blue) and the Mrp2 protein involved in biliary excretion (green). B) Electron micrograph of a slice within a spheroid. C) False colour representation of the distribution of osmium (Os, green) and Ag (red) in a section of spheroid exposed to PVP-coated silver nanoparticles, extracted from nano-XRF data. In all figure panels, the arrows indicate bile canaliculi. n = cell nuclei.
XRF nano-imaging of selected areas of ultra-thin sections of spheroids was performed at beamline ID16B, allowing to identify the structural details of the cell with high spatial resolution, as well as the localisation of AgNP inside the cell (Figure 1). Of the two types of AgNP, XRF imaging showed that cells exposed to cit-AgNP stored excess Ag inside vacuoles in the cell (Figures 2A and B), perhaps to avoid cell toxicity, while cells exposed to PVP-AgNP excreted Ag species into bile canaliculi area through a transmembrane protein known to excrete excess Cu. The Ag salt was observed to accumulate in cells at a much lower level than AgNP and was excreted faster (Figures 2C and D). Moreover, inductively coupled plasma atomic emission spectroscopy (ICP-AES) analyses showed that, after three days of excretion, the fraction of excreted Ag was 65% in the case of the Ag salt, and between 30 and 40% for the two types of AgNP. This means that the dynamic for uptake, transformation, trafficking and excretion is faster upon exposure to dissolved Ag(I) compared to AgNP. These results are also consistent with clinical data that reveals an accumulation of Ag in the livers of long-term hospitalised patients, due to parenteral exposure to AgNP from catheters [1].
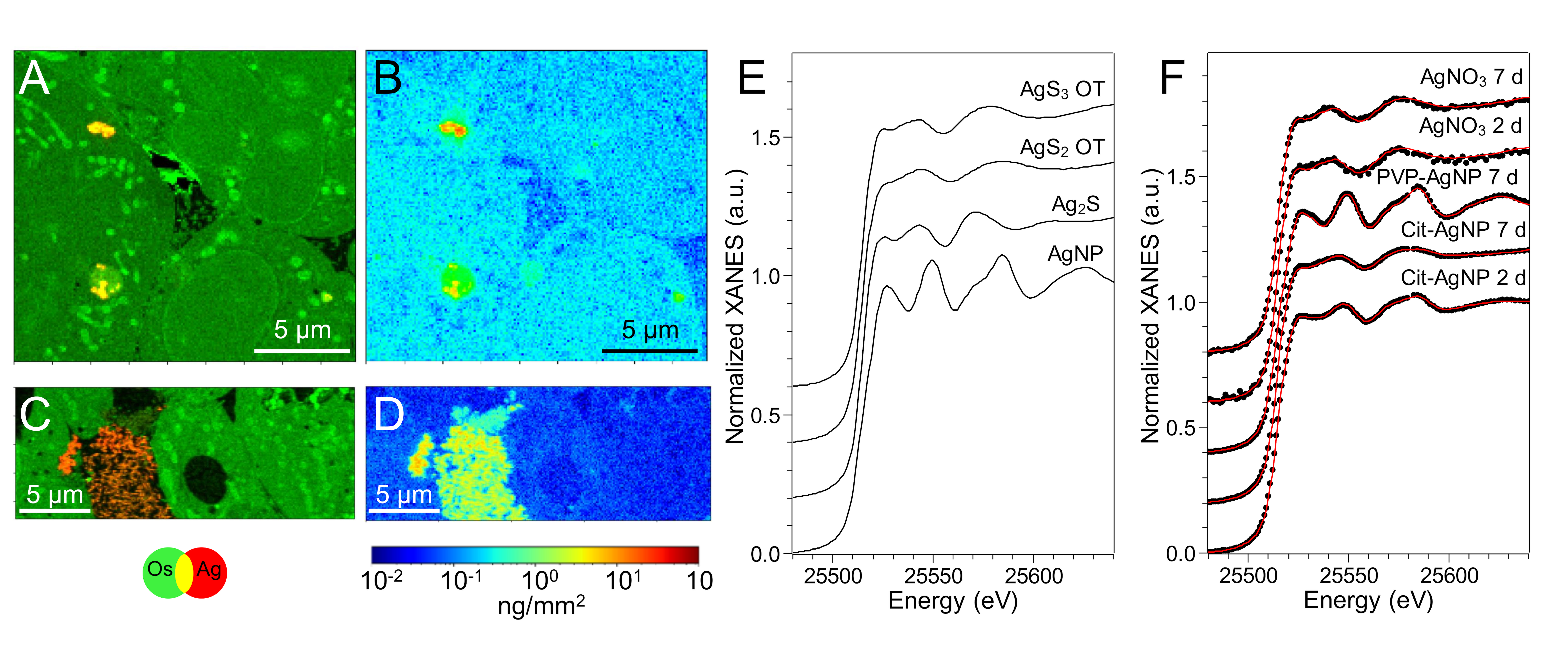
Fig. 2: Nano-XRF imaging data of hepatocyte spheroids exposed for seven days to citrate-coated silver nanoparticles (A,B) or silver salt (AgNO3) (C,D). The overlay between the osmium (Os, green) and Ag (red) signals in (A) and (C) shows the structural details of the interior of the cell with high resolution, while (B) and (D) show the quantitative distribution of the areal density of Ag in logarithmic scale. E) Ag K-edge XANES spectra of reference compounds and (F) of hepatocyte spheroids exposed to citrate- or PVP-coated AgNP, or to AgNO3, for two or seven days. The best fitting curves (red) to the experimental spectra (black circles) are obtained as a linear combination of the reference spectra reported in panel (E).
This study also showed that after two to seven days in cells, AgNP release Ag(I) ions that form compounds mainly with intracellular sulfur. In order to identify the chemical transformations that AgNP and Ag salt undergo in the 3D hepatocyte model, XAS experiments were carried out at beamline BM30, focusing on the X-ray absorption near-edge structure (XANES) region of Ag K-edge XAS spectra and comparing them to the XANES spectra of different reference compounds for inorganic and organic Ag-S species (Figure 2E). The Ag-S species were revealed as inorganic Ag2S and Ag-organothiol (Ag-OT) compounds (Figure 2F), in different proportions depending on the exposure scenario. It has been demonstrated that exposure to slowly released Ag(I) ions from AgNP provokes an efficient cellular response to metal-induced stress [2,3]. The protection mechanism is less efficient in case of fast exposure to Ag(I) ions in a salt.
In conclusion, using a pseudo-organ that reproduces trends observed in vivo and a combination of elemental and structural X-ray nano-imaging and spectroscopy, this study demonstrates that the liver processes AgNP or their transformation products differently depending on the dose and speciation of silver. The findings reveal exposure-dependent mechanisms that can inspire future studies on nanomaterial metabolism in mammals. Moreover, they can help bridge the gap between cellular and animal studies, bringing significant advances to the understanding and risk assessment of the human toxicity of a broadly used nanomaterial.
Principal publication and authors
Deciphering silver nanoparticle fate in liver up to biliary excretion using HepG2/C3A spheroids in scenarios mimicking different exposure pathways, Y. Rekik (a,b), V. Tardillo Suárez (c), V.R. Sharma (a,b), M. Chevallet (a), B. Gallet (d), D. Falconet (e), P. Charbonnier (a), I. Kieffer (f), R. Tucoulou (c), P.-H. Jouneau (b), G. Veronesi (a,c), A. Deniaud (a), Environ. Sci.: Nano 10, 1842-1857 (2023); https://doi.org/10.1039/D3EN00177F
(a) Univ. Grenoble Alpes, CNRS, CEA/IRIG Laboratory of Chemistry and Biology of Metals (CBM), Grenoble (France)
(b) Univ. Grenoble Alpes, CNRS, CEA/IRIG MEM, Grenoble (France)
(c) ESRF
(d) Univ. Grenoble Alpes, CEA, CNRS, Institut de Biologie Structurale (IBS), Grenoble (France)
(e) Univ. Grenoble Alpes, CNRS, INRAE, CEA/IRIG LPCV, Grenoble (France)
(f) BM30 FAME, ESRF, Observatoire des Sciences de l'Univers de Grenoble (France)
References
[1] J. Poznański et al., Int. J. Mol. Sci. 22, 1782 (2021).
[2] G. Veronesi et al., Nanoscale 8, 17012 (2016).
[3] H. Zhang et al., Small 11, 3797 (2015).
| About the beamline: ID16B |
| ID16B is a hard X-ray nanoprobe dedicated to 2D or 3D analysis of heterogeneous materials combining X-ray fluorescence (XRF), diffraction (XRD), absorption spectroscopy (XAS), excited optical luminescence (XEOL), X-ray beam induced current (XBIC) and phase-contrast imaging. Low temperature, in-situ or operando sample environments can be accommodated. ID16B is dedicated to research areas with high scientific and societal impacts such as nanotechnology, earth and environmental sciences, and bio-medical research. |
| About the beamline: BM30 |
| BM30 FAME, the French X-ray absorption spectroscopy (XAS) beamline, covers a wide variety of scientific fields (materials science, biophysics, chemistry) but focuses mainly on Earth sciences, where the speciation of trace elements in heterogeneous and/or complex samples can be studied. FAME’s optical elements have been designed to maximise the photon flux on the sample and to optimise beamline stability and reduce non-statistical noise, in order to permit XAS measurements of diluted samples (ppm concentrations) in very short acquisition times (second). The beamline also offers several sample environments: a low-temperature helium cryostat for measurements on redox sensitive and trace elements, and high-pressure and -temperature autoclaves for in-situ hydrothermal and catalysis studies. The SSHADE/FAME open database (https://www.sshade.eu/db/fame) gathers spectra of standards and characteristic samples, enriched with complete metadata. |