- Home
- News
- Spotlight on Science
- X-ray scattering...
X-ray scattering unearths unexpected Li+ movement in Li-ion batteries
09-10-2023
Wide- and small-angle X-ray scattering computed tomography has been used at beamline ID31 to investigate commercial composite battery anodes. The results reveal new insights into why lithium-ion batteries can fail prematurely, which could help to improve the next generation of batteries.
Predicting how different models of battery will age requires a deep understanding of the degradation mechanisms of each single battery component. This is more difficult when composite electrodes are used, as in the case of graphite-silicon electrodes, where silicon compounds are added to graphite to increase the specific capacity, and hence the energy density, of batteries. In such a case, the lithium ions not only shuffle between the electrodes during battery operation, but there can also be an interaction between the two phases of the composite anode: graphite and silicon-compound. Interestingly, a change in lithiation state (i.e., Li+ relocation) can occur not only during charge and discharge (lithiation and delithiation of the composite electrode), as expected, but also when the battery is kept at Open Circuit Voltage (OCV), that is, when the battery is in a rest state and no current is passing through it.
Tracking the lithiation state of graphite is a straightforward operation with wide-angle X-ray scattering (WAXS), as graphite undergoes a series of changes in phase maintaining the crystalline structure, but silicon lithiation is more complex to monitor, as amorphous phases appear at different lithiation stages. However, the silicon-compound studied (aSi/FeSi2) is composed of nano-domains, which swell during lithiation. This change in volume of the nano-domains provides a clear contrast with the amorphous silicon surrounding the FeSi2 and can be observed in small-angle X-ray scattering (SAXS) data.
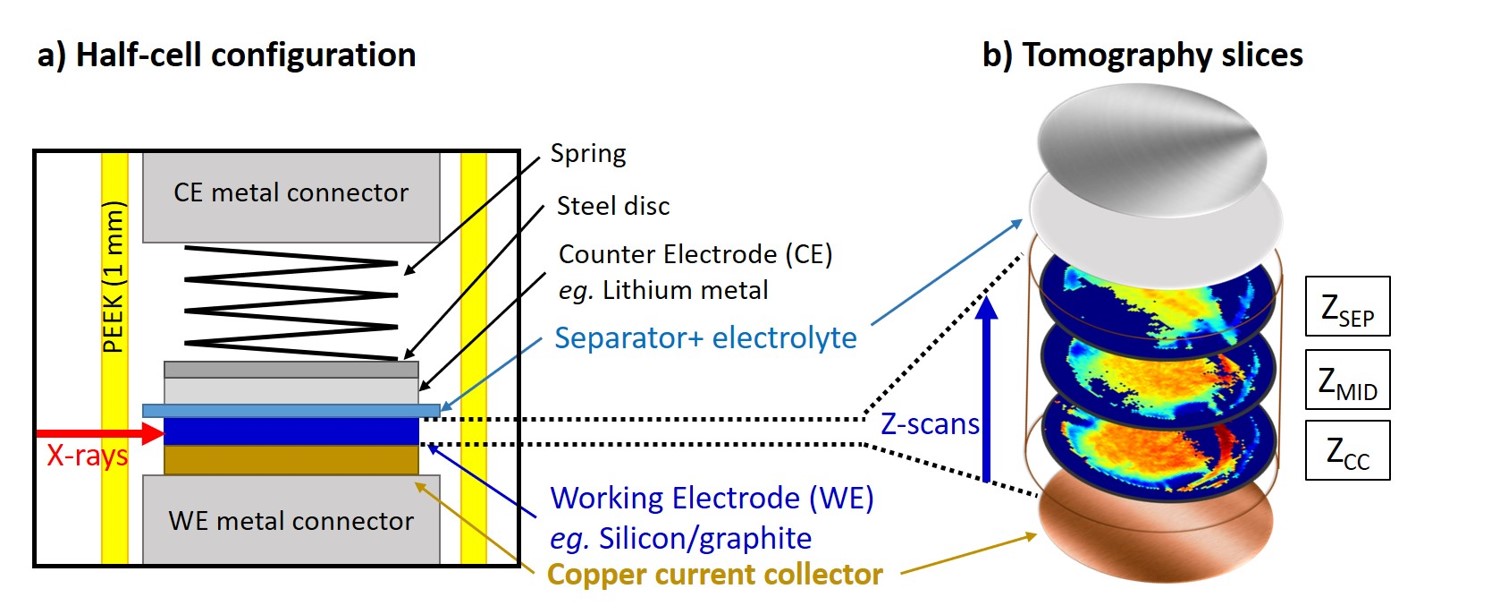
In a collaborative work within the Battery Hub, WAXS and SAXS were used (quasi-)simultaneously at beamline ID31 in a multimodal approach to monitor the lithiation of both graphite and silicon-compounds in a composite electrode. Additionally, the WAXS and SAXS signals were reconstructed using computed tomography (CT), thus obtaining in-plane distribution of the phases in the electrode. A Swagelok-type battery reduced the measurement background and allowed for the aquisition of data in operando mode and at different depths in the electrode (Figure 1).
Click image to enlarge
Fig. 1: a) Schematics of the Swagelok-type battery cell used in this work. The thin PEEK body (1 mm-thick wall) allows for high transmission of the high-energy X-rays (40 keV). The composite working electrode (WE) is placed at the bottom of the cell, on top of which the separator and electrolyte are arranged. Li-metal serves as a counter electrode (CE) and is placed at the top of the stack. A spring in the Swagelok cell serves to hold the pressure on the WE electrolyte-CE stack. b) The visualisation of the sample, highlighting the position at which the tomography scans are acquired within the anode.
The intrinsic heterogeneities of the commercial-like electrodes explain the charge dynamics observed at OCV. Lithium-ion batteries with thick electrodes, which are industrially relevant and have high capacity, exhibit two types of heterogeneity: in-plane heterogeneity, due to the uneven mixing of graphite and silicon compounds in the electrode, and out-of-plane heterogeneity, due to the
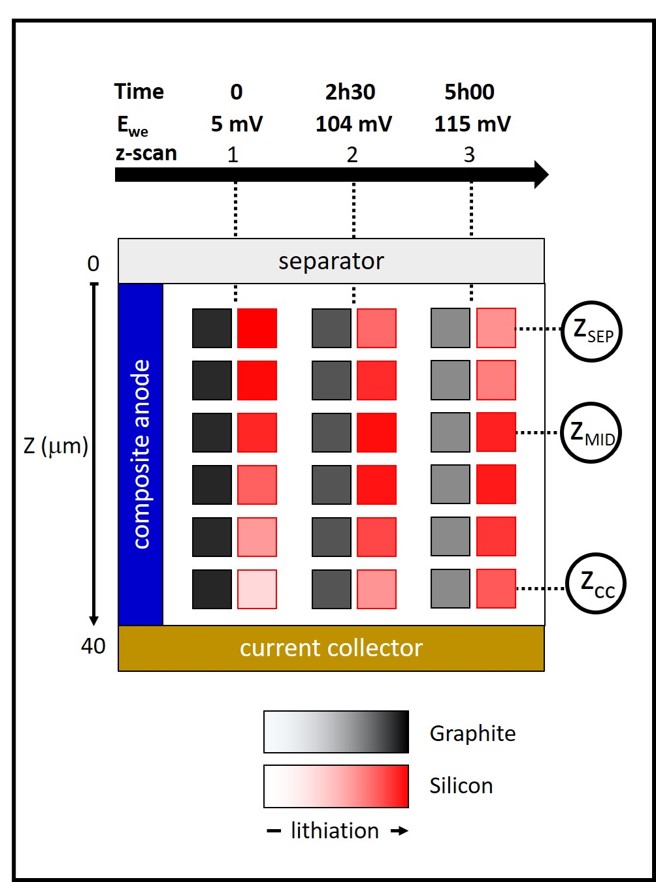
gradual lithiation process that occurs along the depth of the electrode during operation. As a consequence, the composite graphite-silicon electrode at charged (lithiated) state is composed of homogeneously lithiated graphite, while the silicon component is more lithiated close to the separator compared to the current collector. When the battery is stopped in this state, the chemical potential of graphite and silicon at the different depth of the electrode has to re-equilibrate, such that graphite and the highly lithiated silicon close to the separator “donate” Li+ to the poorly lithiated silicon close to the current collector (Figure 2).
Click image to enlarge
Fig. 2: Schematic representation of the electrode dynamic evolution during relaxation. The grey (red) colour bars indicate the amount of lithium in graphite (silicon). From left to right, time dynamics. From top to bottom: depth heterogeneities from separator to current collector.
This study not only highlights the importance of operando measurements to monitor the charge dynamics inside complex systems, but also suggests that industrial computed models used to predict battery ageing are underestimating the degradation process: over-lithiated silicon close to the separator will degrade faster than the silicon close to the current collector, which can accelerate the Li-path degradation through the thick electrode. Considering the differences in lithiation state across the electrode is therefore of foremost importance when predicting ageing of commercial batteries.
Principal publication and authors
Charge dynamics induced by lithiation heterogeneity in silicon-graphite composite anodes, C.L. Berhaut (a) M. Mirolo (b), D. Zapata Dominguez (c), I. Martens (b), S. Pouget (c), N. Herlin-Boime (d), M. Chandesris (e), S. Tardif (c), J. Drnec (b), S. Lyonnard (a), Adv. Energy Mater. 2301874 (2023); https://doi.org/10.1002/aenm.202301874
(a) UGA, CEA, CNRS, IRIG, SyMMES, Grenoble (France)
(b) ESRF
(c) UGA, CEA, IRIG, MEM, Grenoble (France)
(d) UPS, CEA, NIMBE, Gif sur Yvette (France)
(e) UGA, CEA, Liten, Grenoble (France)
| About the beamline: ID31 |
|
Beamline ID31 is dedicated to the investigation of buried interfaces with With a focus on materials facilitating the energy transition, the beamline exploits the high flux and energy provided by ESRF-EBS to probe in operando reactions occurring in real devices (batteries, fuel cells, electrolysers, etc.), |