- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Life Sciences
- Insight into the Regulation of Microtubular Assembly
Insight into the Regulation of Microtubular Assembly
The microtubule cytoskeleton is an essential component of the dynamic architecture of eucaryotic cells. Microtubules serve as guides for the segregation of chromosomes during cell division and constitute tracks along which cellular organelles move. Microtubules are ~ 22 nm diameter hollow tubes whose walls are constituted of parallel protofilaments essentially made of tubulin, a heterodimeric protein constituted of two subunits noted and ß. In order to fulfil their functions, microtubules assemble and disassemble continuously in a way regulated by several families of proteins, among which are the stathmin phosphoprotein family. Proteins of the stathmin family are phosphorylated in response to various extracellular stimuli and have been proposed to serve as relays integrating activated intracellular signalling pathways [1]. All stathmin family proteins share a stathmin-like domain which binds to tubulin to form a 2 tubulin:1 stathmin-like domain ternary complex.
Crystals of a stathmin-like domain:tubulin complex have been obtained. Diffraction data to 4 Å resolution were collected on beamlines ID14-2 and ID14-4. An initial structure of the tubulin molecules of the complex was determined by molecular replacement using as a model the structure of tubulin determined by electron crystallography of planar sheets made of antiparallel protofilaments [2]. Using phases derived from this model, difference electron density maps allowed us to locate a 90 residue stathmin-like a-helix that runs along the complex. The current model of the structure consists of this -helix and two tubulin molecules. It has been refined to an R-factor of 27%. More recently, in a peak-SAD experiment on ID14-4, three seleno-methionine residues (out of ca. 2000 residues in the complex) were located in the stathmin-like
-helix and allow a preliminary assignment of its sequence.
The structure reveals a complex made of a curved head-to-tail assembly of two tubulin molecules, maintained by the stathmin-like a-helix that runs all along it (Figure 15). Two features of the structure deserve notice: i) its curvature resembles that of microtubule oligomeric disassembly products; ii) the tubulin residues that contact stathmin in the complex are identical in the two ß heterodimers. Most interestingly, the spacing along the stathmin-like sequence of the residues that contact tubulin is identical to the spacing of corresponding residues in an internal sequence duplication that has been found in all stathmin family proteins. This strongly suggests that stathmin family proteins have evolved to bind two tubulin molecules and that this is their main function in the cell.
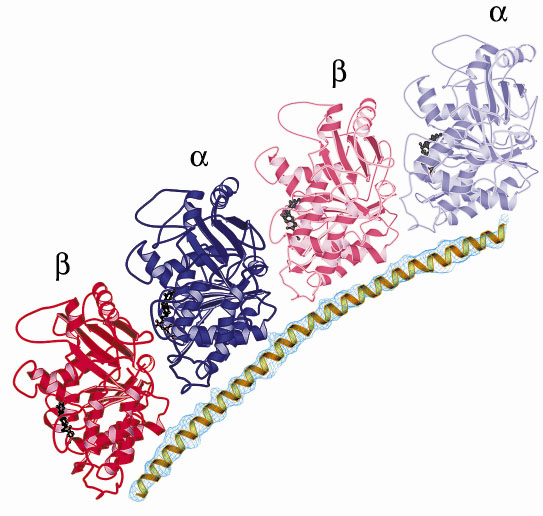 |
| Fig. 15: Ribbon representation of the stathmin-tubulin complex showing the stathmin-like |
Because of its curvature, the stathmin-tubulin complex does not assemble into microtubules. The structure is fully consistent with a sequestering mechanism by which non-phosphorylated stathmin family proteins control microtubule assembly by forming with tubulin a complex that is not incorporated in microtubules. In addition to providing insight into the mechanism of action of stathmin, this study provides an X-ray structure of soluble tubulin. Higher resolution data on this complex may allow rational improvement of anti-cancer drugs that target tubulin.
References
[1] A. Sobel, Trends Biochem. Sci., 16, 301-305 (1991).
[2] E. Nogales, S.G. Wolf and K.H. Downing, Nature, 391, 199-203 (1998).
Principal Publication and Authors
B. Gigant (a), P.A. Curmi (b), C. Martin-Barbey (a), R. Ravelli (c), A. Sobel (b) and M. Knossow (a), Cell, 102, 809-816 (2000).
(a) Laboratoire d'Enzymologie et Biochimie Structurales, C.N.R.S., Gif sur Yvette (France)
(b) INSERM U 440, Institut du Fer à Moulin, Paris (France)
(c) EMBL, Grenoble (France)



