- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2002
- X-ray Imaging
- Waste Incineration: Why is a Synchrotron-based Multidisciplinary Approach Needed?
Waste Incineration: Why is a Synchrotron-based Multidisciplinary Approach Needed?
Review by A. Somogyi (ESRF) and M.C. Camerani Pinzani (Dep. of Inorg. Environm. Chem., Chalmers Univ. of Technology, Göteborg, Sweden)
Finding alternative sources of energy for the decreasing stocks of fossil fuels and dealing with the increasing amount of waste which pollutes the environment are serious problems of our times. Waste incineration appears to be a potential solution for both problems since it has the advantage of transforming the wastes stored chemical energy into electricity or heat while reducing its volume and destroying the chemical reactivity of its organic compounds.
However incineration, as with the combustion of other fuels, creates large quantities of ash containing concentrated amounts of potentially toxic trace metals (i.e. Pb, Ni, Cu, Cd). Thus heat and power technologies based on combustion of such complex fuels require the development of routines for intelligent ash management that make thorough knowledge of the chemical properties of the ash material essential.
The complex study of heavy metal distribution, concentration, speciation and of matrix composition and morphology within individual fly ash particles are of great importance for the evaluation of the possible environmental impact of ash material. This is a valuable complement to the usual bulk analytical methods. The investigation of individual particles is the only possible method to check and develop theoretical models of potential fly ash forming processes during combustion. This might lead to the improvement of the combustion procedure itself through a better control of the transport of heavy metals.
The ID22 multipurpose X-ray microprobe beamline is well-suited for the microscale investigation of such complex materials. The energy of the X-ray beam can be tuned in the 6-70 keV range in order to obtain optimal excitation conditions for the elements to be studied. With the help of different focusing devices e.g. Kirkpatrick-Baez (KB) mirrors (E < 18 keV) and refractive lenses (E > 15 keV), the X-ray source can typically be demagnified to a V x H: 1.5 x (3-10) µm2 spot with 1010-1011 ph/s intensity in the E < 30 keV energy region. Several routinely-available micro-analytical techniques, such as micro X-ray fluorescence analysis (µ-XRF), micro X-ray absorption spectrometry (µ-XAS), micro-diffraction (µ-XRD) and absorption/phase contrast imaging and tomography, make the complex analysis of the sample possible.
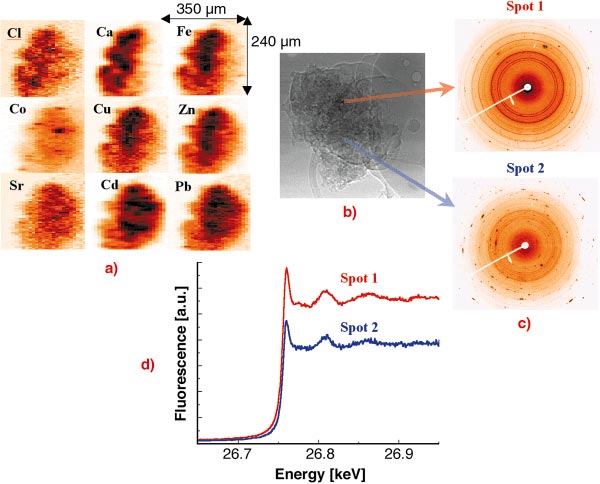
The investigation of individual fly ash particles was performed at 27 keV excitation energy in order to excite the characteristic X-ray lines of heavy metals up to Cd [1,2]. The beam was focused by compound refractive lenses (CRL) to a V x H = 2 x 10 µm2 spot. In order to investigate the spatial elemental distribution and inter-elemental correlation within single particles and the dependence of the heavy metal content on the particle size, several particles of different sizes were raster scanned in the focused beam while registering the XRF spectra in each voxel. The obtained 2D characteristic X-ray intensity maps of a large particle can be seen in Figure 101a. The variation of the crystalline structure of the matrix was measured simultaneously in each voxel by collecting XRD WAXS spectra using a large area CCD detector (see Figure 101c) in order to study the possible attachment of the investigated elements to a given crystalline structure.
 |
|
Fig. 101: Investigation of a single municipal waste fly ash particle by different micro X-ray analytical methods. (a) 2D X-ray fluorescence maps, step size: H x V: 2 x 10 mm2; (b) absorption image registered by a high- resolution CCD camera; (c) X-ray diffraction patterns registered by a large area CCD; (d) Extended XANES spectra measured in the chosen spots of the particle. |
The dissolution and transport of metal ions from the ash matrix to soil water are key steps because dissolved ions are available for biological uptake and ground water contamination. Thus the toxicity of ash strongly depends on the chemical speciation of the different potentially toxic elements. The chemical speciation of a given element in a voxel chosen on the basis of the 2D XRF/XRD maps can be investigated by micro-XAS. The extended XANES spectra of the element to be investigated (see Figure 101d) is obtained by changing the energy of the monochromatic excitation X-ray beam in the vicinity of the absorption edge while registering the intensity of its characteristic X-ray intensity (fluorescence mode). The obtained XAS spectra provide information about the oxidation state and first shell neighbours of the element.
The eventual fate of heavy metals is also influenced by their position within the particle: on the surface they are more prone to leaching while at some depth within the matrix they might be more shielded from chemical attack by water. Due to the large penetration depth of high energy X-rays the 2D intensity maps (see Figure 101a) only reflect the variation of the overall concentration among the irradiated voxels but do not give any information about the internal elemental distribution. These internal elemental distributions can be reconstructed by appropriate mathematical algorithms from X-ray sinograms obtained by successive linear scans and rotations of a slice of the sample (X-ray fluorescence tomography). X-ray absorption tomography can be used to obtain information about the particle morphology (3D linear absorption coefficient distribution), usually determined from light, non-detectable elements.
Some important conclusions can be drawn from the study of waste fly ash particles about the chemical properties of their Cd-content [3]. In most of the cases the main Cd-bearing phase was the Ca-containing matrix. Cd identified in this study was found to be evenly distributed throughout all the particle sizes investigated. X-ray fluorescence tomography showed that Cd was distributed not only on the surface but also within the matrix of the investigated slices. Comparing XAS spectra of fly ashes and reference compounds showed that in the particles studied Cd is present in the oxidation state +2. Analysis of linear combinations of standard spectra allows us to estimate the fly-ash composition as an admixture of CdSO4, CdO and CdCl2 in all the analysed spots.
From an environmental perspective, these results are of particular importance when considering the short-term leaching potential of fly ashes. The fact that Cd appears in an easily soluble form in a soluble particle matrix (Ca) can make the direct dumping of fly ashes problematic but on the other hand it might allow a leaching medium to concentrate Cd-containing phases before dumping the major fraction of ash material. It is expected that the knowledge of the spatial elemental distribution and speciation of different heavy metals, as obtained in the present study for Cd, would allow formulation of models for the formation of fly ash particles during the combustion process. These models may be used as a basis to improve Fluidised Bed Combustion processes by including the control of the transport of heavy metals.
The technique presented here is applicable to fly ash from all sorts of fuel, like coal, sludges, biomass, peat and their mixtures, as well as from municipal solid wastes.
References
[1] M.C. Camerani, A. Somogyi, M. Drakopoulos, B.-M. Steenari, Spectrochimica Acta Part B 56, 1355-1365 (2001).
[2] M.C. Camerani Pinzani, A Somogyi, B. Vekemans, A.S. Simionovici, B.M. Steenari, I. Panas, submitted to Environmental Science and Technology (2002).
[3] M.C. Camerani Pinzani, A. Somogyi, A. Simionovici, S. Ansell, B.M. Steenari and O. Lindquist, Environmental Science and Technology 36 (14), 3165 3169 (2002).



