- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2003
- X-ray Imaging
- High-resolution X-ray Imaging of Vascular Networks
High-resolution X-ray Imaging of Vascular Networks
There is growing interest in high-resolution imaging of vascular structures for different physiological or pathological problems [1]. In the context of brain vascular imaging, in-vivo synchrotron tomography has permitted the measurement of contrast agent concentration and local blood flow for the whole brain of a rat with the unprecedented spatial resolution of 350 micrometres [2]. This resolution still remains inadequate compared with the few micrometre scale of small vessels. Since 50% of the vessel diameters in the cerebral cortex are distributed between 3 to 10 micrometres, micrometre resolution is necessary to get important information about the vascular network at the scale of small capillaries. Even if synchrotron X-rays offer such an opportunity, a specific biological sample preparation is needed. A contrast agent concentration much larger than those of CT-scan angiography has to be injected into the vascular network. Moreover, the total vascular relative volume is only 3 to 5% in either human or rat brain. Hence, the effective vascular contribution to X-ray absorption or phase contrast represents a cumulated thickness of a few tens of micrometres only, along the few millimetres of the total X-ray path inside the tissue. This is why we have developed a sample preparation specific to synchrotron high-resolution X-ray tomography that has permitted the visualisation of the micro-vascular structure of the brain's cortical grey matter. The X-ray absorption inside the sample is due to the cumulated contribution of the epoxy-resin around and inside the biological sample and the injected solution inside the vascular network. The histograms shown in Figure 131 have been obtained from the three-dimensional attenuation maps of barium injected samples for an X-ray energy of 20 keV and a sample-detector distance D = 13 mm at the ID19 beamline. This histogram shows two well-separated peaks. The first one is associated with the epoxy-resin, while the second one corresponds to contrast-agent absorption in the vessels. Volume renderings of the resulting 3D images are shown in Figure 132a and 132b, which are maximum-intensity projections of barium injected samples. The vessel density observed on these images illustrates the quality of the preparation, while the apparent high vessel density of the rather loose vascular network results from the projection over a one-millimetre thickness. These renderings also demonstrate the quality of the local vessel contrast obtained with the highest barium concentration (600 mg/ml). The views show columnar structures that spread through the grey matter depth from the pial surface. Those renderings are, to our knowledge, the first visualisation of the vascular structure of the cortex's grey matter over its entire thickness, on the micrometre scale. The quantitative analysis of the 3D images will provide important morphometric information, of interest to different areas where the micro-vascularisation plays a key role such as physiological and pathological angiogenesis and brain functional imaging whose basic principles rely on cortical micro-vascularisation.
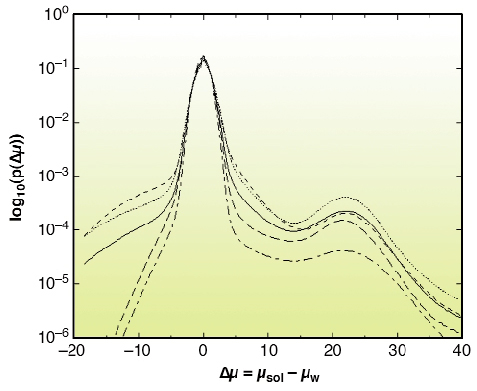 |
|
Fig. 131: Normalised histograms p( |
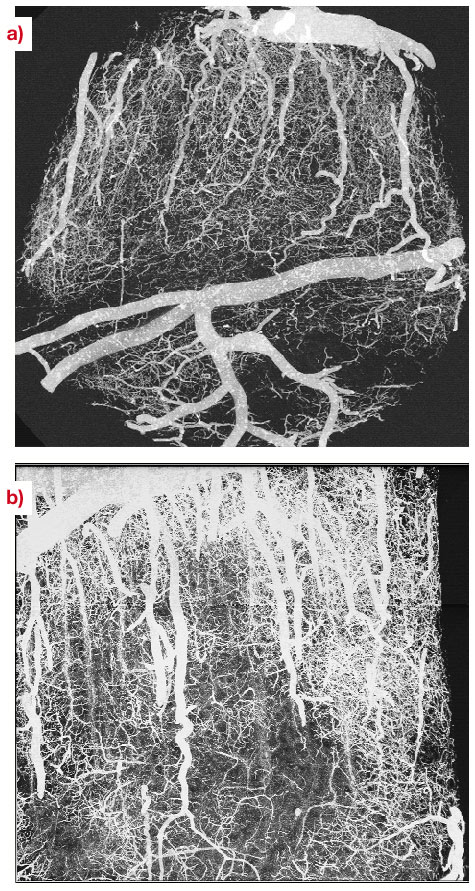 |
|
Fig. 132: Volume renderings of a rat brain cortical sample injected with barium (600mg/ml), obtained at 20 keV in absorption mode with a voxel size equal to 1.4 micrometre. Maximum intensity projections have been performed for volumes with a size equal to |
References
[1] A.S. Popel, A.R. Pries, and D.W. Laaf, J. Neuro. Meth, 111, 911-913, (1998); R. Weissleder and U. Mahmood, Radiology, 219, 316-333, (2001).
[2] H. Elleaume, A.M.. Charvet, S. Corde, F. Estève and J.F. Le Bas, Physics in Medecine & Biology, 47, 3369-3385, (2002).
Principal publication and Authors
F. Plouraboué (a), P. Cloetens (b), C. Fonta (c), A. Steyer (d), F. Lauwers (e) and J-P Marc-Vergnes (e), submitted to J. Microsc. (2003).
(a) IMFT, UMR 5502, Toulouse (France)
(b) ESRF, Grenoble, (France)
(c) CERCO, UMR 5549, Toulouse, (France)
(d) Université de Paris I, (France)
(e) INSERM U455, Toulouse, (France)



