- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- X-ray Absorption and Magnetic Scattering
- A structural-reactive rollercoaster: palladium nanoparticles during redox cycling
A structural-reactive rollercoaster: palladium nanoparticles during redox cycling
Supported noble metals, such as Pd, are widely used as heterogeneous catalysts; indeed most of us use such catalysts everyday within the exhaust system of our cars. These catalysts are designed to ensure that any toxic by products of the internal combustion engine (unburnt hydrocarbons, nitrogen oxides, and CO) do not reach the atmosphere at large and are instead converted efficiently to N2, CO2, and water. To achieve this, the catalysts have to be able to work within a rapidly changing “redox” (reduction/oxidation) environment; the changes being controlled to maximise efficiency by the car’s engine management system.
Whilst this technological approach has existed for sometime, exactly what happens to the catalysts from a structural-reactive point of view within this cycling is far from being understood. Furthermore these catalysts, and similar ones used in a variety of industries, are still plagued with problems such as particle sintering, which limit their useful lifetime. Sintering causes initially small and well-separated metallic catalyst particles to agglomerate and form larger species. This has two effects: firstly the active surface area of the metal component is massively reduced; and secondly the very chemistry displayed by the much bigger particles may be radically different (and often unwanted) relative to the much smaller starting particles.
Recently we have carried out an experiment designed to permit the internal working of catalysts during redox cycling to be studied with accurate time-resolution, and from three synchronous approaches: structural (in this case dispersive EXAFS, ID24); the global measurement of performance (mass spectrometry); and a time-resolving probe of the species that exist transiently on the surface of the catalyst during the cycling (infrared spectroscopy).
Our findings are that the structure (size/shape) of the supported Pd nanoparticles appears to be very fluid indeed, changing very rapidly in response to the changes in composition of the reactive feedstock. There were indications that oxidation of the Pd nanoparticles was relatively slow compared to their structural rearrangement and this even in the presence of relatively large amount of O2.
 |
|
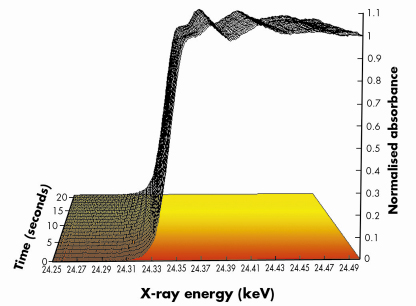
Fig. 99: Normalised Pd K edge energy dispersive EXAFS spectra derived during a switch from a 5%NO/He feed to a 5%CO/He feed (both 75mlmin–1 flow) at 673 K over a 1wt%Pd/10ZCA sample. Each spectrum was acquired in ca. 250 msecs. For clarity only 1 spectrum/second is plotted. The red to yellow shading is indicative of the switch from the NO to CO feed. |
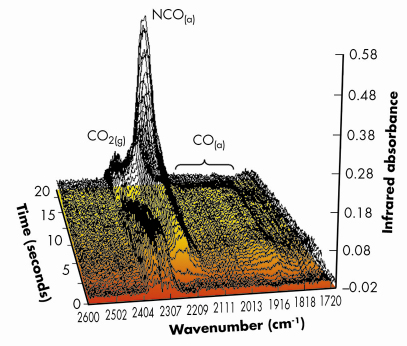
Figures 99 and 100 shows results typical of the observed behaviour from both XAFS and infrared perspectives for a 1wt%Pd/10ZCA sample (10ZCA = 10% zirconia/ceria/alumina). The dynamic behaviour of the surface functionality is clearly delineated by the infrared component of the experiment allowing us to follow the formation and removal of a number of different species with, in the current case, a temporal resolution of 300 milliseconds. From this we can identify a number of carbonyl species forming and reacting; isocyanate (NCO) species forming on the Pd but migrating to the oxide where they decompose; the formation of CO2 in the gas phase; and a number of other species such as adsorbed NO on Pd and carbonate species associated with the support material.
 |
|
Fig. 100: Infrared spectra (averaged over 9 reversible NO/CO switches) taken at the same time as the EXAFS spectra shown in Figure 99, collected with a 3 Hz spectral acquistion speed. The major gas phase and surface functionalities observed during the experiment are indicated. Again the shading of the flow of the graph indicates the change of reactive feedstock. |
At the same time the dispersive XAFS lets us see how the Pd responds to the dynamism exhibited by its feedstock. Both the near edge (XANES) and extended XAFS (EXAFS) spectra indicate that the size and the shape of the Pd particles varies rapidly and reversibly with the changes in environment: the Pd agglomerates under a reducing (CO) environment and disperses to smaller/flatter particles under oxidising (NO) conditions, moreover, these techniques also allowed us to determine that oxidation of the Pd component (to for instance PdO) is much slower than the aforementioned structural rearrangements. Under these circumstances therefore, it is these more subtle, and apparently more facile, size and shape changes that have the potential to play a significant role in the catalytic chemistry being displayed by the operating catalyst rather than the other, more well-known processes, such as oxidation.
Quantification of the precise nature of the structure reactivity by means of a variety of types of measurement paints a picture of a massively responsive and dynamic nano-world. Therein the variables of starting metal particle size, temperature and the redox potential of the gas phase are braided together in complex and sensitively interdependent ways that can have a massive bearing on their cumulative performance as functional materials.
Principal publication and authors
M.A. Newton (a), C. Belver (b), A. Martínez-Arias (b), M. Fernández-García (b), Nature Mat. 6, 528-532 (2007); Angew. Chem Int. Ed. 45, 8629-8631 (2007).
(a) ESRF
(b) Instituto de Catalisis y Petroleoquímica, Madrid (Spain)



