- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- X-ray Absorption and Magnetic Scattering
- Long-lasting controversies on properties of matter at high pressure: the role of XAS
Long-lasting controversies on properties of matter at high pressure: the role of XAS
The thermodynamic variable pressure is being increasingly employed to study the behaviour of matter when atoms are brought closer together. This allows experimental verification of the different models used to describe the structural and the electronic properties of matter. If diffraction methods are still the predominant high pressure techniques for structural studies involving synchrotron radiation, X-ray absorption spectroscopy (XAS) offers a complementary description of matter under pressure: it gives a local picture since it probes the local environment around the photoabsorber atom with chemical selectivity. It is also sensitive to pressure-induced electronic transitions since it probes the electronic density of states above the Fermi level. Here we show two examples that highlight the complementarity of XAS with respect to other more conventional techniques in the understanding of fundamental issues in condensed matter physics.
Metallic zinc, together with its electronic counterpart metallic cadmium, are intriguing systems among divalent hexagonal close-packed structured metals as the c/a axial ratio exceeds about 14-15% the ideal value for hcp structures. This anomaly makes many of its solid state properties highly anisotropic and, at high pressure, Zn is believed to undergo a Lifshitz or electronic topological transition (ETT) near 10 GPa. This type of phase transition arises when distortion of the electronic band structure due to an external agent such as doping, hydrostatic pressure or anisotropic strain modifies the topology of the Fermi surface. For this reason, compressed zinc has been extensively studied both theoretically and experimentally with a disconcerting plethora of different results. In particular X-ray diffraction on Zn under pressure has proven to be particularly sensitive to hydrostatic conditions [1]. We have studied compressed metallic zinc using energy dispersive XAS at ID24 in different hydrostatic conditions. The aim of this study was to gain direct evidence for the ETT in solid Zn under pressure by probing the variation of the X-ray absorption near-edge structure (XANES). In fact, according to the electric dipole selection rules, Zn K-edge XANES directly probes the density of unoccupied states with p symmetry. Therefore, the variations as a function of pressure of the near-edge structure of the spectra are a probe for pressure-induced changes in the electronic density of states above the Fermi level and may give direct evidence of the ETT. Figure 101 shows the near-edge part of the spectra acquired using various pressure transmitting media, 4:1 methanol:ethanol and neon, to provide different hydrostatic conditions. Methanol:ethanol is known to yield quasihydrostatic conditions only up to 10 GPa, whereas previous experiments suggest that the strength of solid Ne above 100 GPa is sufficiently low that the material can be used as a quasihydrostatic medium over this pressure range. The gradual evolution of the XANES features with pressure for the two experiments carried out in different hydrostatic conditions not only show no evidence of an electronic transition in Zn under pressure, but also demonstrate that XAS is much less sensitive to nonhydrostaticity.
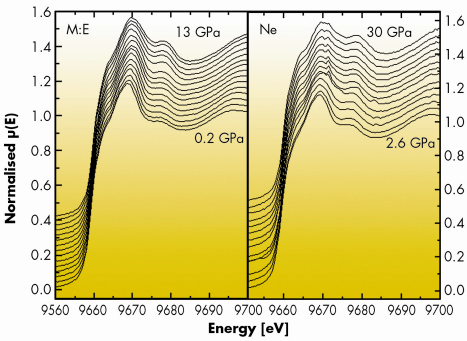 |
|
Fig. 101: XANES spectra for metallic Zn measured using 4:1 methanol:ethanol (left panel) and Ne (right panel) as pressure transmitting media. |
Besides the sensitivity to changes in electronic structure, the chemical selectivity of XAS can be used to give an insight complementary to diffraction in the determination of chemical order in high pressure structures. Knowledge of local chemical order is of capital importance because with increasing density, the relative weight of different energetic terms in play are modified, and it is often difficult to foresee how and to what extent this occurs. Therefore, being able to tell for example whether or not atoms A are predominantly surrounded by atoms B in an AB alloy, allows one to test thermodynamical and structural models derived using theoretical methods. The octect compounds A(n)B(n–1) are a class of compounds extensively studied at high pressure using X-ray diffraction. In particular the increased popularity of angle dispersive X-ray diffraction has led to a re-evaluation of the high pressure phase diagram of this class of materials and to the identification of Cmcm as a common structure at high pressure [2]. Using XAS we have been able to verify that the high pressure Cmcm phase of gallium phosphide is locally chemically ordered, when X-ray diffraction could only conclude on the presence of long-range disorder. Ga K-edge XANES spectra measured on beamline ID24 were compared to state-of-the-art full multiple scattering calculations (Figure 102) and confirm unequivocally the occurrence of a Cmcm symmetry with a high degree of local chemical ordering. This means that, notwithstanding the low ionicity of this compound, this parameter still dictates short range interactions even at very high densities.
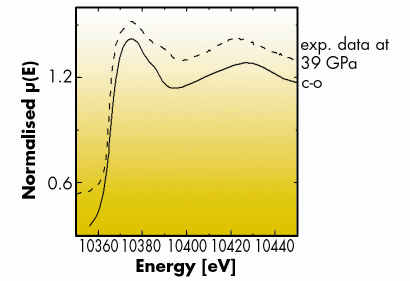 |
|
Fig. 102: From bottom to top: Simulated Ga K-edge spectrum of GaP in a chemically ordered Cmcm structure (c-o) and experimental data at 39 GPa (dashed line). |
References
[1] K. Takemura, Phys.Rev. B, 60, 6171-6174 (1999).
[2] R. J. Nelmes, M.I. McMahon, S.A. Belmonte, Phys. Rev. Lett. 79, 3668-3671 (1997).
Principal publication and authors
G. Aquilanti (a), A. Trapananti (a), M. Minicucci (b), F. Liscio (c), A. Twaróg (d), E. Principi (b), S. Pascarelli (a), Phys. Rev. B, 76, 144102(6) (2007); G. Aquilanti (a), H. Libotte (e), W.A. Crichton (a), S. Pascarelli (a), A. Trapananti (a), J.-P. Itié (f), Phys. Rev. B, 76, 64103(7) (2007).
(a) ESRF
(b) CNISM, CNR-INFM, Università degli Studi di Camerino (Italy)
(c) Laboratoire de Thermodynamique et Physico-Chimie Métallurgiques, ENSEEG, Saint Martin d’Hères (France)
(d) Faculty of Physics, Warsaw University of Technology (Poland)
(e) Département de Physique, Université de Liège (Belgium)
(f) Synchrotron Soleil, Gif-sur-Yvette (France)



