- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- Structural biology
- An open and shut case
An open and shut case
Bacteria are survivors and cope with dramatic changes in environment during their life cycle. One such stress is osmotic shock, in moving from a salty environment (such as biological fluids) to a salt-free environment (water), whereby bacteria experience pressures up to 14 atm. The pressure is felt as a swelling of the cytoplasm (turgor pressure), and, unless relieved, the consequence is rupture of the membrane. However, bacteria are able to release this pressure build up using the mechanosensitive channels, MscS and MscL.
The breakthrough in the structural understanding of mechanosensitive channels came from the work of the Rees lab [1]. This 3.95 Å structure showed that MscS is a heptamer with each monomer consisting of three transmembrane helices at the
N-terminus and a large cytoplasmic domain. The heptamer is arranged symmetrically around a 7-fold symmetry axis normal to the membrane. One of the transmembrane helices (TM3) is split into two TM3a and TM3b. Two rings of leucine side chains on TM3a point into the central channel. This provides a “vapour lock” and, in essence, these side chains prevent any ions or proteins from travelling from the cytoplasm into the periplasm. Under pressure this lock must be opened, however the mechanism by which this is accomplished remained obscure.
The route to structural insight came from identifying mutants which were more likely to be open from electrophysiology studies.
Thus we set out to study the crystal structures of different mutants of MscS to see whether any showed an altered state. The project would have been impossible without the ESRF facilities, membrane protein crystals do not diffract well and we screened many hundreds of crystals from several mutants before finally arriving at a 3.45 Å structure for the A106V mutant. This mutation appears to destabilise the previously observed closed form but does not appear to stabilise the open form. Compared to the native structure, the mutant MscS shows dramatic changes in the position and orientation of transmembrane helices, most notably TM1 and TM2. These two helices have moved around 1/7 of a revolution and increased their tilt with respect to the membrane (Figure 64). As a result of this dramatic movement, TM3a pivots at G113 in such a way that it become parallel to the membrane normal. It is also “pulled” out of the central pore.
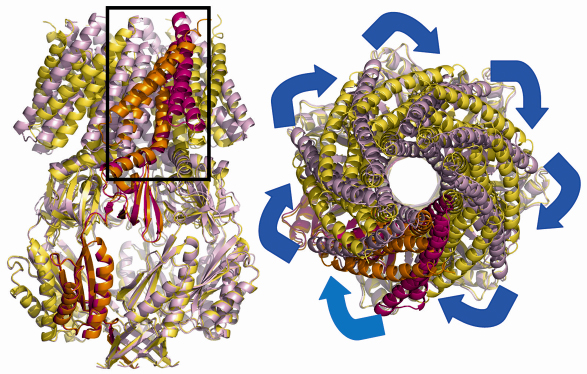 |
|
Fig. 64: The open structure of MscS is shown in orange (A subunit) and yellow (subunits B-G). The closed structure is shown in red (A subunit) and pink (subunits B-G). TM1 and TM2 have rotated around the seven-fold axis. |
The movement of TM3a results in a iris like motion of the leucine side chains which create an open pore. Thus a picture emerges of TM1 and TM2 acting as the pressure sensor which in turn operates the valve on TM3a. The structural data of course are only a model for the situation inside the cell. The structural model for the opening and closing of MscS predicts a number of key side chain interactions. These hypotheses were tested by site directed mutagenesis and gave strong support to the structural model.
The significance of our work lies in understanding the physiology of pathogens; the opening and closing of channels is an important topic in biology. The second significant finding is that carefully chosen site directed mutants may provide an important route to the observation of conformational states of membrane proteins.
Principal publication and authors
W. Wang (a), S.S. Black (b), M.D. Edwards (b), S. Miller (b), E.L. Morrison (b), W. Bartlett (b), C. Dong (a), J.H. Naismith (a) and I.R. Booth (b), Science 321, 1179 (2008).
(a) Centre for Biomolecular Sciences, University of St. Andrews (UK)
(b) School of Medical Sciences, University of Aberdeen (UK)
Reference
[1] R.B. Bass, P. Strop, M. Barclay, D.C. Rees, Science 298, 1582 (2002).



