- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- X-ray imaging
- The chemical processes behind Van Gogh’s discolouring flowers
The chemical processes behind Van Gogh’s discolouring flowers
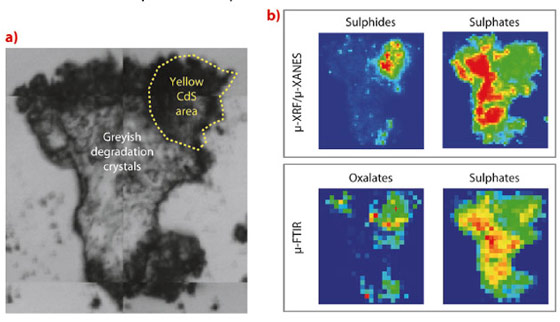
In the past, we elucidated the chemical process that caused the fading of the bright yellow colour on a painting by James Ensor, a contemporary of Vincent Van Gogh. For this Ensor case, experiments at ESRF beamlines ID18F and ID21 demonstrated that the discolouration was caused by photo-oxidation of the original cadmium yellow pigment (CdS) to colourless cadmium sulphate (CdSO4). In this work, a similar case was studied: the formation of a disturbing crust on the cadmium yellow paint strokes of a painting by Van Gogh. As Figure 65 illustrates, a superimposed film of opaque greyish crystals completely obscures the original warm yellow colour of the underlying paint. In addition, the case was rendered more complex due to the presence of a varnish coating that was applied on top of the degraded paint during a previous conservation treatment, most probably in an attempt to consolidate the affected paint.
Two samples were extracted from this degraded paint and analysed at beamlines ID21 and ID18F and the P06 beamline of PETRA III at DESY. The combination of a number of complementary species-selective analytical techniques (i.e. µ-XRF/µ-XRD, µ-XRF/µ-XANES and µ-FTIR) revealed the presence of a thin (< 10 µm) film at the interface between the bulk CdS paint and the overlying varnish, containing cadmium oxalate, cadmium carbonate and lead sulphate (Figure 66). The identification of these compounds on the micrometre level enabled an explanation of the chemical pathway that led to the discolouration phenomenon observed at the macroscopic level.
In spite of its divergent morphology, we concluded that the alteration on the Van Gogh still life was initially caused by oxidation of the original CdS pigment, similar to the Ensor case. However, for the Van Gogh case, secondary reactions took place between the primary degradation product (CdSO4) and lead- and oxalate- ions present in the varnish. Upon its application, the solvent rich varnish would have dissolved the CdSO4 and dispersed this degradation product throughout the coating. It appears that the freed Cd2+ ions reprecipated out with C2O42- instead of SO42-. Oxalate ions are commonly observed in cultural heritage materials and are in this case believed to be a degradation product of the varnish/paint medium. The sulphate anions, for their part, found a suitable reaction partner in lead ions which resulted in the formation of anglesite (PbSO4) crystals. Most probably, the Pb2+ ions were supplied by a lead-based drier, a hypothesis that was corroborated by the fact that anglesite (PbSO4) was also identified in the Ensor case [1].
The origin of the encountered cadmium carbonate was less unequivocal. It may have been formed as a secondary degradation product following the primary photo-degradation of CdS or perhaps by the capture of atmospheric CO2, as suggested by Mass et al. [2]. Alternatively, a tertiary process involving the further breakdown of cadmium oxalate into cadmium carbonate cannot be excluded while finally its presence might also be explained as simply a residue of the original raw material used for the industrial production of CdS.
In conclusion, this study showed how the primary degradation process, i.e. the oxidation of the original cadmium sulphide to colourless cadmium sulphate, was followed by secondary reactions after the application of a varnish film. Not only did this coating dissolve the highly soluble cadmium sulphate, but it also supplied reaction partners (i.e. oxalate and lead ions) for the resulting Cd2+ and SO42- ions. In this way, the application of what was once assumed to be a protective coating eventually led to the formation of a disfiguring film at the paint/varnish interface instead.
Principal publication and authors
G. Van der Snickt (a), K. Janssens (a), J. Dik (b), W. de Nolf (a), F. Vanmeert (a), J. Jaroszewicz (a), M. Cotte (c,d), G. Falkenberg (e) and L. Van der Loeff (f), Anal. Chem. 84, 10221-10228 (2012).
(a) University of Antwerp (Belgium)
(b) Delft University of Technology (The Netherlands)
(c) Laboratoire d’archéologie moléculaire et structurale, CNRS UMR 8220 (France)
(d) ESRF
(e) DESY (Germany)
(f) Kröller-Müller Museum (The Netherlands)
References
[1] G. Van der Snickt, J. Dik, M. Cotte, K. Janssens, J. Jaroszewicz, W. De Nolf, J. Groenewegen and L. Van der Loeff, Anal. Chem. 81, 2600-2610 (2009).
[2] Jennifer Mass, personal communication, July 2012.