Chrome Yellows
Degradation of chrome yellow pigments
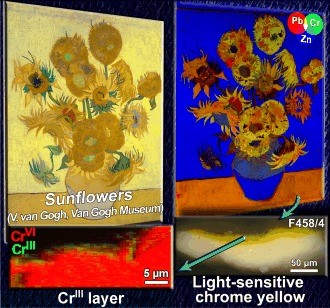
Chrome yellow pigments have been invented during the 19th Century and can have different colors and names, which are due to different synthesis processes. Not only their composition, but also their stability vary. Experiments carried out ID21 were performed both on fragments from major paintings (such as the Van Gogh’s sunflowers) and on different sets of model samples to assess the effects of pigment composition, light, humidity, binder, etc.
|
L. Monico, M. Cotte, F. Vanmeert, L. Amidani, K. Janssens, G. Nuyts, J. Garrevoet, G.Falkenberg, P. Glatzel, A. Romani, and C. Miliani, "Damages Induced by Synchrotron Radiation-Based X-ray Microanalysis in Chrome Yellow Paints and Related Cr-Compounds: Assessment, Quantification, and Mitigation Strategies", Analytical Chemistry, 14164-14173 , (2020) |
|
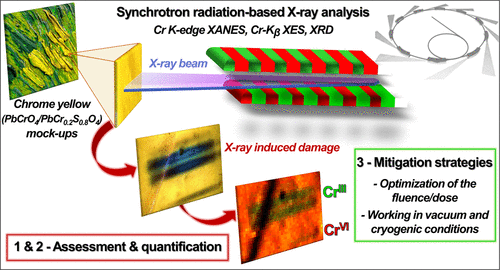
Synchrotron radiation (SR)-based X-ray methods are powerful analytical tools for several purposes. They are widely used to probe the degradation mechanisms of inorganic artists’ pigments in paintings, including chrome yellows (PbCr1–xSxO4; 0 ≤ x ≤ 0.8), a class of compounds often found in Van Gogh masterpieces. However, the high intensity and brightness of SR beams raise important issues regarding the potential damage inflicted on the analyzed samples. A thorough knowledge of the SR X-ray sensitivity of each class of pigment in the painting matrix is therefore required to find analytical strategies that seek to minimize the damage for preserving the integrity of the analyzed samples and to avoid data misinterpretation. Here, we employ a combination of Cr K-edge X-ray absorption near-edge structure spectroscopy, Cr-Kβ X-ray emission spectroscopy, and X-ray diffraction to monitor and quantify the effects of SR X-rays on the stability of chrome yellows and related Cr compounds and to define mitigation strategies. We found that the SR X-ray beam exposure induces changes in the oxidation state and local coordination environment of Cr ions and leads to a loss of the compound’s crystalline structure. The extent of X-ray damage depends on some intrinsic properties of the samples (chemical composition of the pigment and the presence/absence and nature of the binder). It can be minimized by optimizing the overall fluence/dose released to the samples and by working in vacuum and under cryogenic conditions.
|
L. Monico, E. Hendriks, M. Geldof, C. Miliani, K. Janssens, B. Brunetti, M. Cotte, F. Vanmeert, A. Chieli and G. van der Snickt, "Chemical Alteration and Colour Changes in the Amsterdam Sunflowers", (2019). |
|
L. Monico, L. Sorace, M. Cotte, W. de Nolf, K. Janssens, A. Romani and C. Miliani, "Disclosing the Binding Medium Effects and the Pigment Solubility in the (Photo) reduction Process of Chrome Yellows (PbCrO4/PbCr1–x S x O4)", ACS Omega, 4, 6607-6619 (2019). |
The darkening due to chemical alteration of chrome yellows (PbCrO4/PbCr1–xSxO4) is a phenomenon threatening a large number of 19th–20th century paintings, including the Amsterdam Sunflowers by Vincent van Gogh. Our earlier studies have proven that the alteration is due to a Cr(VI) → Cr(III) reduction with Cr(V)-species that are formed as long-lived intermediates and that PbCr1–xSxO4 (0 < x ≤ 0.8) types undergo reduction more readily than monoclinic, S-free, PbCrO4. In this context, there is still lack of knowledge about the effects of the chemical properties of the binding medium (i.e., chemical composition and drying process) and the solubility of chrome yellows on the overall reduction pathways. Here, we study a series of naturally and photochemically aged mock-up paints prepared by mixing chrome yellow powders (PbCrO4/PbCr0.2S0.8O4) with either linseed oil or a water-based acrylic emulsion as the binding medium. Equivalent paints made up of the highly soluble K2CrO4 were also investigated and used as benchmarks to provide a more in-depth understanding of the influence of the solubility on the chromate reduction pathways in the two different binders. A combination of synchrotron radiation-based Cr K-edge X-ray absorption near edge structure (XANES), electron paramagnetic resonance (EPR), and UV–Visible spectroscopy measurements shows that: (1) the Cr(VI) reduction results from the interaction between the pigment and the binder; (2) the process is more significant in oil, giving rise to Cr(V)- and Cr(III)-species as well as oxidized organic compounds; (3) the lightfastness of the chrome yellow pigment is enhanced in the acrylic binder; and (4) the tendency toward chromium reduction increases with increasing solubility of the pigment. Based on our findings, we propose a scheme for the mechanism of the (photo) reduction process of chrome yellows in the oil and acrylic binder. Overall, our results provide new insights into the factors driving the degradation of lead chromate-based paints in artworks and contribute to the development of strategies for preserving them over time.
 |
V. Otero, M. Vilarigues, L. Carlyle, M. Cotte, W. De Nolf and M. J. Melo, "A little key to oxalate formation in oil paints: protective patina or chemical reactor?", Photochemical & Photobiological Sciences (2018). |
By means of synchrotron based techniques, we propose an integrated mechanism for the degradation of 19thcentury chrome yellow oil paints based on pigment reconstructions from historical recipes. We show that for certain paint formulations the darkening of these colours is triggered by the binder photodegradation which leads to the formation of calcium oxalate at the expense of the filler CaCO3, and the reduction of the chrome yellow pigment (Cr6+/Cr3+). Considering that calcium oxalate is formed as a thin superficial layer, that may prevent light absorption by the paint bulk, we discuss its role as protective patina.
 |
L. Monico, K. Janssens, M. Cotte, L. Sorace, F. Vanmeert, B. G. Brunetti and C. Miliani, "Chromium speciation methods and infrared spectroscopy for studying the chemical reactivity of lead chromate-based pigments in oil medium", Microchemical Journal, 124, 272-282 (2016). |
Environmental factors, such as light, humidity and temperature are triggering agents for the alteration of organic and/or inorganic constituents of oil paintings. The oxidation of the organic material is favored by increasing of relative humidity and temperature, whereas processes involving changes of the oxidation states of a number of inorganic pigments (e.g., vermilion, cadmium yellows, zinc yellows, chrome yellows) are mainly activated by light-exposure.
In view of the optimization of the long-term conservation and restoration strategies of paintings it is of relevant interest to establish the consequences of thermal parameters (temperature and relative humidity) on the chemical/photochemical-reactivity and the nature of the alteration products of light sensitive-pigments in oil medium.
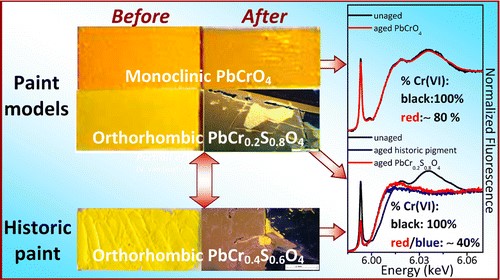
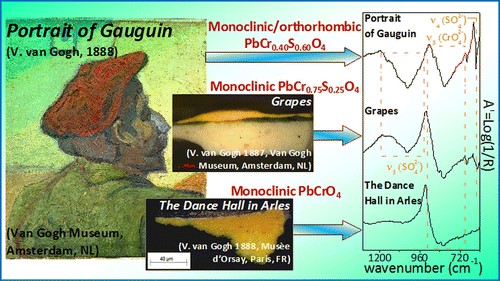
To this aim here we propose a multi-method analytical approach based on the combination of diffuse reflectance UV–Vis, FTIR, synchrotron radiation (SR)-based micro X-ray fluorescence (μ-XRF)/micro-X-ray absorption near edge structure (XANES) and electron paramagnetic resonance (EPR) spectroscopies for studying the effects of different relative humidity conditions before and after light exposure on the reactivity of a series of lead chromate-based pigments [such as PbCrO4∙ PbO (monoclinic), PbCrO4 (monoclinic) and PbCr0.2S0.8O4 (orthorhombic)] in an oil medium. The investigation of paint models was also compared to that of a late 19th century historical orthorhombic PbCr0.4S0.6O4 oil paint.
Diffuse reflectance UV–Vis and FTIR spectroscopies were used to obtain information associated with chromatic changes and the formation of organo-metal degradation productsat the paint surface. SR-based Cr K-edge μ-XANES/μ-XRF mapping analysis and EPR spectroscopy were employed in a complementary fashion to determine the amount, nature and distribution of Cr(III) and Cr(V)-based alteration compounds within the paints with micrometric spatial resolution.
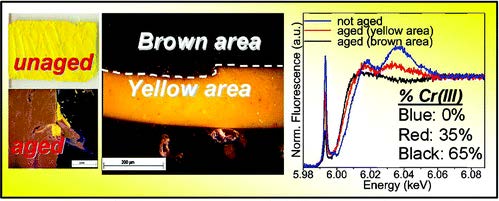
Under the employed thermal aging conditions, lead(II)-carboxylates and reduced Cr-compounds (in abundance of up to about 35% at the surface) have been identified in the lead chromate-based paints. The tendency of chromates to become reduced increased with increasing moisture levels and was favored for the orthorhombic PbCr0.2S0.8O4 compounds. The redox process gave rise to the formation of Cr(V)-species in relative amount much higher than that was formed in the equivalent paint which was exposed only to light.
After light-exposure of the thermally aged paints, compounds ascribable to the oxidation of the organic binder were detected for all the types of pigments. Nevertheless, the previous thermal treatment increased the tendency toward photo-reduction of only the PbCr0.2S0.8O4 pigment. For this light-sensitive compound, the thickness variation of the reduced Cr-rich (ca. 70%) photo-alteration layer with moisture levels could be ascribed to a surface passivation phenomenon that had already occurred before photochemical aging.
|
L. Monico, K. Janssens, M. Alfeld, M. Cotte, F. Vanmeert, C. G. Ryan, G. Falkenberg, D. L. Howard, B. G. Brunetti and C. Miliani, "Full spectral XANES imaging using the Maia detector array as a new tool for the study of the alteration process of chrome yellow pigments in paintings by Vincent van Gogh", Journal of Analytical Atomic Spectrometry, 30, 613-626 (2015). |
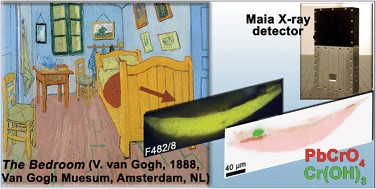
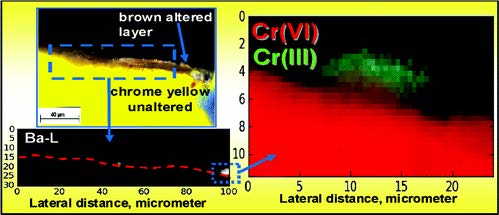
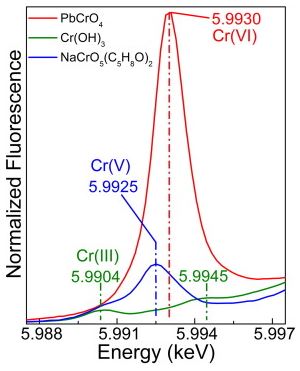
A combination of synchrotron radiation (SR) micro X-ray fluorescence (μ-XRF) and XRF mode X-ray absorption near edge structure (XANES) measurements at the Cr K-edge already allowed us to establish that the photo-reduction of chromates to Cr(III) compounds is the cause of darkening of chrome yellow pigments (PbCr1−xSxO4, 0 ≤ x ≤ 0.8) in a number of paintings by Vincent van Gogh and in corresponding artificially aged paint models. A silicon drift detector (SDD) was employed to record the Cr-K XRF radiation in these X-ray micro beam-based measurements. However, in view of the limited count rate capabilities and collection solid angle of a single device, μ-XRF and μ-XANES employing single element SDDs (or similar) are primarily suited for collection of spectral data from individual points. Additionally, collection of XRF maps via point-by-point scanning with relatively long dwell times per point is possible but is usually confined to small areas. The development of the 384 silicon-diode array Maia XRF detector has provided valuable solutions in terms of data acquisition rate, allowing for full spectral (FS) XANES imaging in XRF mode, i.e., where spectroscopic information is available at each pixel in the scanned map. In this paper, the possibilities of SR Cr K-edge FS-XANES imaging in XRF mode using the Maia detector are examined as a new data collection strategy to study the speciation and distribution of alteration products of lead chromate-based pigments in painting materials. The results collected from two micro-samples taken from two Van Gogh paintings and an aged paint model show the possibility to perform FS-XANES imaging in practical time frames (from several minutes to a few hours) by scanning regions of sample sizes of the same order (more than 500 μm). The sensitivity and capabilities of FS-XANES imaging in providing representative chemical speciation information at the microscale (spatial resolution from ∼2 to 0.6 μm) over the entire scanned area are demonstrated by the identification of Cr(OH)3, Cr(III) sulfates and/or Cr(III) organometallic compounds in the corresponding phase maps, as alteration products. Comparable Cr-speciation results were obtained by performing equivalent higher spatial resolution SR μ-XRF/single-point μ-XANES analysis using a more conventional SDD from smaller regions of interest of each sample. Thus, large-area XRF mode FS-XANES imaging (Maia detector) is here proposed as a valuable and complementary data collection strategy in relation to “zoomed-in” high-resolution μ-XRF mapping and single-point μ-XANES analysis (SDD).
 |
L. Monico, K. Janssens, M. Cotte, A. Romani, L. Sorace, C. Grazia, B. G. Brunetti and C. Miliani, "Synchrotron-based X-ray spectromicroscopy and electron paramagnetic resonance spectroscopy to investigate the redox properties of lead chromate pigments under the effect of visible light", Journal of Analytical Atomic Spectrometry, 30, 1500-1510 (2015). |
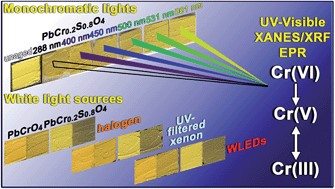
Light-induced redox processes have been established as the cause of the chromatic alterations of a number of artists' pigments used from the 15th to the 20th century. Despite the fact that a general comprehension of the mechanisms has been provided through the characterization of photo-degraded compounds, both exhaustive information on the wavelength-dependence of the alteration process of the pigments and experimental evidence in how visible light may influence the formation pathways of specific secondary compounds are still lacking. Establishing an analytical protocol for the study of wavelength-dependence of pigments on photo-redox pathways is relevant for the safe illumination of paintings, especially in view of the possible use of spectrally tunable light sources such as white light emitting diodes (WLEDs). In this work, we propose an integrated approach based on a combination of diffuse reflectance UV-visible, synchrotron radiation (SR)-based micro X-ray fluorescence (μ-XRF)/X-ray absorption near edge structure (μ-XANES) and electron paramagnetic resonance (EPR) spectroscopies to study the photo-redox process of Cr(VI) → Cr(III) for lead chromate yellows (PbCr1−xSxO4, 0 ≤ x ≤ 0.8) under exposure to different monochromatic light. In view of the thin (3–5 μm) alteration layer that is formed at the paint surface after light exposure, SR-based Cr K-edge μ-XANES/μ-XRF analysis was employed to obtain information on the abundance, nature and distribution of the alteration of Cr(III)-compounds at the micrometric-scale level. On the other hand, EPR spectroscopy was used as a complementary tool to the SR-based X-ray methods due to its sensitivity for revealing species containing one or more unpaired electrons and for distinguishing different coordination geometries of paramagnetic centers, such as Cr(V)-species. Semi-quantitative indications about the darkening of the paint surface were obtained by UV-Vis spectroscopy. An abundance of reduced Cr down to around 50% was detected at the aged surface of chrome yellow paints. The reduction process was favored not only by wavelengths shorter than 460 nm (i.e., where the pigment shows its maximum absorption) but also by light in the 490–530 nm range. The first evidence of the presence of Cr(V)-intermediates in the Cr(VI) → Cr(III) reduction reaction allowed the risks of inducing photo-degradation of the 490–530 nm wavelength range to be explained.
 |
L. Monico, K. Janssens, E. Hendriks, F. Vanmeert, G. Van der Snickt, M. Cotte, G. Falkenberg, B. G. Brunetti and C. Miliani, "Evidence for Degradation of the Chrome Yellows in Van Gogh’s Sunflowers: A Study Using Noninvasive In Situ Methods and Synchrotron‐Radiation‐Based X‐ray Techniques", Angewandte Chemie, 127, 14129-14133 (2015). |
This paper presents firm evidence for the chemical alteration of chrome yellow pigments in Van Gogh’s Sunflowers (Van Gogh Museum, Amsterdam). Noninvasive in situ spectroscopic analysis at several spots on the painting, combined with synchrotron‐radiation‐based X‐ray investigations of two microsamples, revealed the presence of different types of chrome yellow used by Van Gogh, including the lightfast PbCrO4 and the sulfur‐rich PbCr1−xSxO4 (x≈0.5) variety that is known for its high propensity to undergo photoinduced reduction. The products of this degradation process, i.e., CrIII compounds, were found at the interface between the paint and the varnish. Selected locations of the painting with the highest risk of color modification by chemical deterioration of chrome yellow are identified, thus calling for careful monitoring in the future.
|
L. Monico, K. Janssens, F. Vanmeert, M. Cotte, B. G. Brunetti, G. Van der Snickt, M. Leeuwestein, J. Salvant Plisson, M. Menu and C. Miliani, "Degradation Process of Lead Chromate in Paintings by Vincent van Gogh Studied by Means of Spectromicroscopic Methods. Part 5. Effects of Nonoriginal Surface Coatings into the Nature and Distribution of Chromium and Sulfur Species in Chrome Yellow Paints", Analytical Chemistry, 26, 10804-10811 (2014). |
|
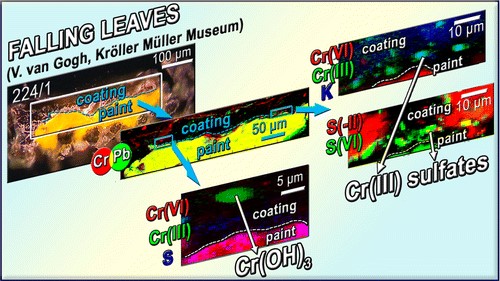
The darkening of lead chromate yellow pigments, caused by a reduction of the chromate ions to Cr(III) compounds, is known to affect the appearance of several paintings by Vincent van Gogh. In previous papers of this series, we demonstrated that the darkening is activated by light and depends on the chemical composition and crystalline structure of the pigments. In this work, the results of Part 2 are extended and complemented with a new study aimed at deepening the knowledge of the nature and distribution of Cr and S species at the interface between the chrome yellow paint and the nonoriginal coating layer. For this purpose, three microsamples from two varnished paintings by Van Gogh and a waxed low relief by Gauguin (all originally uncoated) have been examined. Because nonoriginal coatings are often present in artwork by Van Gogh and contemporaries, the understanding of whether or not their application has influenced the morphological and/or physicochemical properties of the chrome yellow paint underneath is relevant in view of the conservation of these masterpieces. In all the samples studied, microscopic X-ray fluorescence (μ-XRF) and X-ray absorption near edge structure (μ-XANES) investigations showed that Cr(III)-based alteration products are present in the form of grains inside the coating (generally enriched of S species) and also homogeneously widespread at the paint surface. The distribution of Cr(III) species may be explained by the mechanical friction caused by the coating application by brush that picked up and redistributed the superficial Cr compounds, likely already present in the reduced state as result of the photodegradation process. The analysis of the XANES profiles allowed us to obtain new insights into the nature of the Cr(III) alteration products, that were identified as sulfate-, oxide-, organo-metal-, and chloride-based compounds. Building upon the knowledge acquired through the examination of original paint samples and from the investigation of aged model paints in the last Part 4 paper, in this study we aim to characterize a possible relation between the chemical composition of the coating and the chrome yellow degradation pathways by studying photochemically aged model samples covered with a dammar varnish contaminated with sulfide and sulfate salts. Cr speciation results did not show any evidence of the active role of the varnish and added S species on the reduction process of chrome yellows.
|
L. Monico, K. H. Janssens, C. Miliani, G. Van der Snickt, B. G. Brunetti, M. Cestelli Guidi, M. Radepont and M. Cotte, "The Degradation Process of Lead Chromate in paintings by Vincent van Gogh studied by means of Spectromicroscopic methods. Part IV: Artificial ageing of model samples of co-precipitates of lead chromate and lead sulfate", Analytical Chemistry, 85, 860-867 (2013). |
Previous investigations about the darkening of chrome yellow pigments revealed that this form of alteration is attributable to a reduction of the original Cr(VI) to Cr(III), and that the presence of sulfur-containing compounds, most often sulfates, plays a key role during this process. We recently demonstrated that different crystal forms of chrome yellow pigments (PbCrO4 and PbCr1–xSxO4) are present in paintings by Vincent van Gogh. In the present work, we show how both the chemical composition and the crystalline structure of lead chromate-based pigments influence their stability. For this purpose, oil model samples made with in-house synthesized powders of PbCrO4 and PbCr1–xSxO4 were artificially aged and characterized. We observed a profound darkening only for those paint models made with PbCr1–xSxO4, rich in SO42– (x ≥ 0.4), and orthorhombic phases (>30 wt %). Cr and S K-edge micro X-ray absorption near edge structure investigations revealed in an unequivocal manner the formation of up to about 60% of Cr(III)-species in the outer layer of the most altered samples; conversely, independent of the paint models’ chemical composition, no change in the S-oxidation state was observed. Analyses employing UV–visible diffuse reflectance and Fourier transform infrared spectroscopy were performed on unaged and aged model samples in order to obtain additional information on the physicochemical changes induced by the aging treatment.
|
L. Monico, K. H. Janssens, C. Miliani, B. G. Brunetti, M. Vagnini, F. Vanmeert, G. Falkenberg, A. M. Abakumov, Y. Lu, H. Tian, J. Verbeeck, M. Radepont, M. Cotte, E. Hendriks, M. Geldof, L. Van der Loeff, J. Salvant and M. Menu, "The Degradation Process of Lead Chromate in paintings by Vincent van Gogh studied by means of Spectromicroscopic methods. Part III: Synthesis, characterization and detection of different crystal forms of the chrome yellow pigment", Analytical Chemistry, 85, 851-859 (2013). |
|
The painter, Vincent van Gogh, and some of his contemporaries frequently made use of the pigment chrome yellow that is known to show a tendency toward darkening. This pigment may correspond to various chemical compounds such as PbCrO4 and PbCr1-xSxO4, that may each be present in various crystallographic forms with different tendencies toward degradation. Investigations by X-ray diffraction (XRD), mid-Fourier Transform infrared (FTIR), and Raman instruments (benchtop and portable) and synchrotron radiation-based micro-XRD and X-ray absorption near edge structure spectroscopy performed on oil-paint models, prepared with in-house synthesized PbCrO4 and PbCr1-xSxO4, permitted us to characterize the spectroscopic features of the various forms. On the basis of these results, an extended study has been carried out on historic paint tubes and on embedded paint microsamples taken from yellow-orange/pale yellow areas of 12 Van Gogh paintings, demonstrating that Van Gogh effectively made use of different chrome yellow types. This conclusion was also confirmed by in situ mid-FTIR investigations on Van Gogh’s Portrait of Gauguin (Van Gogh Museum, Amsterdam).
|
L. Monico, G. Van der Snickt, K. Janssens, W. De Nolf, C. Miliani, J. Dik, M. Radepont, E. Hendriks, M. Geldof and M. Cotte, "Degradation Process of Lead Chromate in Paintings by Vincent van Gogh Studied by Means of Synchrotron X-ray Spectromicroscopy and Related Methods. 2. Original Paint Layer Samples", Analytical Chemistry, 83, 1224-1231 (2011). |
|
The darkening of the original yellow areas painted with the chrome yellow pigment (PbCrO4, PbCrO4·xPbSO4, or PbCrO4·xPbO) is a phenomenon widely observed on several paintings by Vincent van Gogh, such as the famous different versions of Sunflowers. During our previous investigations on artificially aged model samples of lead chromate, we established for the first time that darkening of chrome yellow is caused by reduction of PbCrO4 to Cr2O3·2H2O (viridian green), likely accompanied by the presence of another Cr(III) compound, such as either Cr2(SO4)3·H2O or (CH3CO2)7Cr3(OH)2 [chromium(III) acetate hydroxide]. In the second part of this work, in order to demonstrate that this reduction phenomenon effectively takes place in real paintings, we study original paint samples from two paintings of V. van Gogh. As with the model samples, in view of the thin superficial alteration layers that are present, high lateral resolution spectroscopic methods that make use of synchrotron radiation (SR), such as microscopic X-ray absorption near edge (μ-XANES) and X-ray fluorescence spectrometry (μ-XRF) were employed. Additionally, μ-Raman and mid-FTIR analyses were carried out to completely characterize the samples. On both paint microsamples, the local presence of reduced Cr was demonstrated by means of μ-XANES point measurements. The presence of Cr(III) was revealed in specific areas, in some cases correlated to the presence of Ba(sulfate) and/or to that of aluminum silicate compounds.
|
L. Monico, G. Van der Snickt, K. Janssens, W. De Nolf, C. Miliani, J. Verbeeck, H. Tian, H. Tan, J. Dik, M. Radepont and M. Cotte, "Degradation Process of Lead Chromate in Paintings by Vincent van Gogh Studied by Means of Synchrotron X-ray Spectromicroscopy and Related Methods. 1. Artificially Aged Model Samples", Analytical Chemistry, 83, 1214-1223 (2011). |
|
On several paintings by artists of the end of the 19th century and the beginning of the 20th Century a darkening of the original yellow areas, painted with the chrome yellow pigment (PbCrO4, PbCrO4·xPbSO4, or PbCrO4·xPbO) is observed. The most famous of these are the various Sunflowers paintings Vincent van Gogh made during his career. In the first part of this work, we attempt to elucidate the degradation process of chrome yellow by studying artificially aged model samples. In view of the very thin (1−3 μm) alteration layers that are formed, high lateral resolution spectroscopic methods such as microscopic X-ray absorption near edge (μ-XANES), X-ray fluorescence spectrometry (μ-XRF), and electron energy loss spectrometry (EELS) were employed. Some of these use synchrotron radiation (SR). Additionally, microscopic SR X-ray diffraction (SR μ-XRD), μ-Raman, and mid-FTIR spectroscopy were employed to completely characterize the samples. The formation of Cr(III) compounds at the surface of the chrome yellow paint layers is particularly observed in one aged model sample taken from a historic paint tube (ca. 1914). About two-thirds of the chromium that is present at the surface has reduced from the hexavalent to the trivalent state. The EELS and μ-XANES spectra are consistent with the presence of Cr2O3·2H2O (viridian). Moreover, as demonstrated by μ-XANES, the presence of another Cr(III) compound, such as either Cr2(SO4)3·H2O or (CH3CO2)7Cr3(OH)2 [chromium(III) acetate hydroxide], is likely.