- Home
- Users & Science
- Find a beamline
- X-ray nanoprobe
- ID21 - X-ray Microscopy Beamline
- Cultural heritage activities at ID21
- Understanding degradation phenomena and assessing conservation treatments
- Mercury sulfides (cinnabar and vermillion)
Mercury sulfides (cinnabar and vermillion)
Degradation of mercury sulfide pigments (vermillion and cinnabar)
The most common degradation of red mercury sulfide, used in a cinnabar natural form or in a vermilion synthetic form, is turning into black or silver-gray. This phenomenon is rather capricious and does not happen systematically. Since Antiquity, cinnabar was often used in paintings, even if it was known to suffer from sunlight and humidity degradation. However, the degradation process is far from being completely understood. Furthermore, several mechanisms seem to be involved in this blackening, leading to different chemical species. Up to recent times, the most advanced hypothesis was the phase transformation of red hexagonal cinnabar into black cubic metacinnabar by action of light. Yet, chlorine was shown to be possibly involved in the darkening of natural cinnabar and in the formation of white and gray crusts in vermilion paintings. Experiments carried out at ID21 aimed at understanding the composition of black and grey layers covering vivid red paintings from Pompeii, Pedralbes and “The Adoration of the Magi” (1617–1618) by P. P. Rubens. Experiments were also conducted on model samples to assess the effect of several environmental factors on HgS stability.
 |
M. Radepont, Y. Coquinot, K. Janssens, J.-J. Ezrati, W. de Nolf and M. Cotte, "Thermodynamic and experimental study of the degradation of the red pigment mercury sulfide", Journal of Analytical Atomic Spectrometry, 30, 599-612 (2015). |
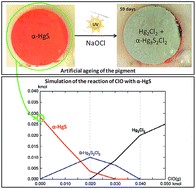
The red pigment mercury sulfide, called cinnabar or vermilion, is well known to suffer from an alteration giving rise to a grey, grey-white or black color at the surface of degraded works of art. This phenomenon can dramatically affect the esthetical value of artworks. This work aims at assessing the factors (light, halides) influencing the instability of red mercury sulfide and understanding (by combining thermodynamic and experimental approaches) the chemical equilibria governing the formation and evolution of the different degradation compounds. From the thermodynamic study of the Hg–S–Cl–H2O system, it was concluded that Hg(0), Hg3S2Cl2, and Hg2Cl2 can be formed from the reaction of α-HgS with ClO(g). In the second part, the artificial ageing experiments presented were carried out on model samples following the conditions assessed in the first part, in order to reproduce natural ageing observed on red mercury sulfide. Similarly to degradation compounds detected on original works of art, mercury chlorine compounds such as calomel (Hg2Cl2) and corderoite (α-Hg3S2Cl2) were identified on the surface of α-HgS model samples, when exposed to light and a sodium hypochlorite solution. Sulfates were detected as well, and more particularly gypsum (CaSO4·2H2O) when Ca was originally present in the model sample. The relationship between color and composition is discussed as well.
 |
M. Radepont, W. de Nolf, K. Janssens, G. Van der Snickt, Y. Coquinot, L. Klaassen and M. Cotte, "The use of microscopic X-ray diffraction for the study of HgS and its degradation products corderoite ([small alpha]-Hg3S2Cl2), kenhsuite ([gamma]-Hg3S2Cl2) and calomel (Hg2Cl2) in historical paintings", Journal of Analytical Atomic Spectrometry, 26, 959-968 (2011). |
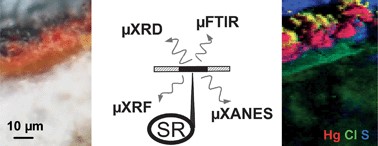
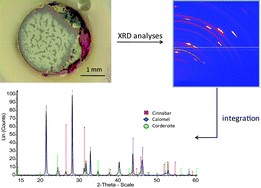
Since antiquity, the red pigment mercury sulfide (α-HgS), called cinnabar in its natural form or vermilion red when synthetic, was very often used in frescoes and paintings, even if it was known to suffer occasionally from degradation. The paint hereby acquires a black or silver-grey aspect. The chemical characterization of these alteration products is rather challenging mainly because of the micrometric size and heterogeneity of the surface layers that develop and that are responsible for the color change. Methods such as electron microscopy, synchrotron-based microscopic X-ray fluorescence, microscopic X-ray absorption near edge spectroscopy, Raman microscopy and secondary ion microscopy have been previously employed to identify the (Hg- and S-) compounds present and to study their co-localization. Next to these, also microscopic X-ray diffraction (XRD) (either by making use of laboratory X-ray sources or when used at a synchrotron facility) allows the identification of the crystal phases that are present in degraded HgS paint layers. In this paper we employ these various forms of micro-XRD to analyze degraded red paint in different paintings and compare the results with other X-ray based methods. Whereas the elemental analyses of the degradation products revealed, next to mercury and sulfur, the presence of chlorine, X-ray diffraction allowed the identification, next to α-HgS, of the Hg and S-containing compound calomel (Hg2Cl2) but also of the Hg, S and Cl-containing minerals corderoite (α-Hg3S2Cl2) and kenhsuite (γ-Hg3S2Cl2). These observations are consistent with X-ray absorption spectroscopymeasurements performed at the S- and Cl-edges.
 |
M. Cotte and J. Susini, "Watching Ancient Paintings through Synchrotron-Based X-Ray Microscopes", Mrs Bulletin, 34, 403-405 (2009). |
|
|
M. Cotte, J. Susini, V. A. Solé, Y. Taniguchi, J. Chillida, E. Checroun and P. Walter, "Applications of synchrotron-based micro-imaging techniques to the chemical analysis of ancient paintings", Journal of Analytical Atomic Spectrometry, 23, 820-828 (2008). |
Ancient paintings are complex materials in terms of chemical analysis because they are usually made of organic/mineral, amorphous/crystallized, major/minor mixtures, evolving with time, and organized in micrometric multi-layered arrangements. In this context, synchrotron micro-imaging techniques offer a powerful analytical platform to reveal the two dimensional atomic, molecular and structural compositions of such complex systems, at a micrometre resolution. The two selected examples illustrate the two main concerns of restorers and conservators: looking backwards, to get insight into ancient artistic practices (in particular through the identification of pigments and binders in Bamiyan Buddhist mural paintings); and looking forward, to preserve works of art as long as possible (through a better understanding of cinnabar blackening in Medieval Spanish paintings). From the analytical chemistry point of view, they also illustrate the relevance of combining micro X-ray fluorescence, micro X-ray absorption spectroscopy, micro X-ray diffraction, and micro-FTIR for the complete analysis of painting cross-sections (binders and pigments).
 |
M. Cotte, J. Susini, N. Metrich, A. Moscato, C. Gratziu, A. Bertagnini and M. Pagano, "Blackening of Pompeian Cinnabar Paintings: X-ray Microspectroscopy Analysis", Analytical Chemistry, 78, 7484-7492 (2006). |
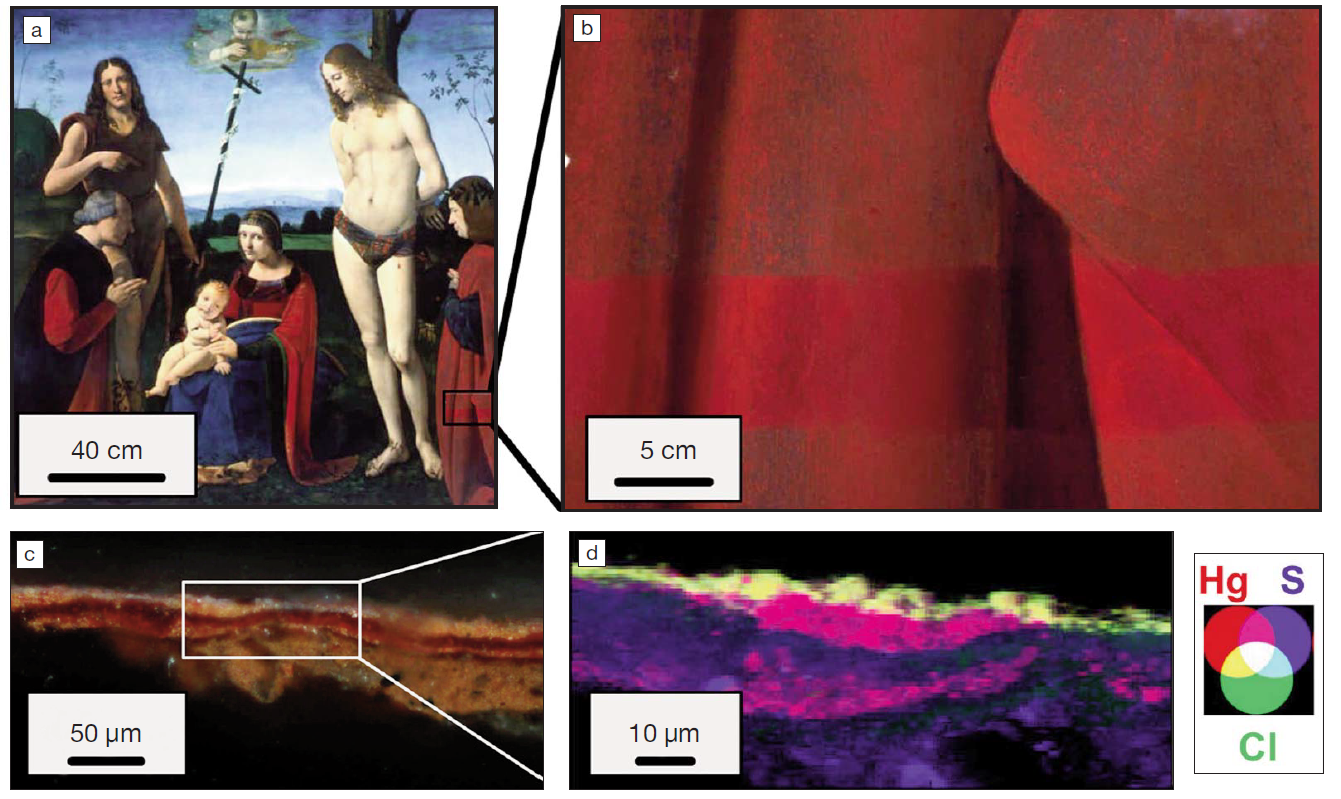
Red Pompeian paintings, very famous for their deep intensity, are currently suffering from darkening. The origins of this darkening degradation are not clearly identified yet and remain a major issue for curators. In the specific case of cinnabar (HgS)-based red pigment, a photoinduced conversion into black metacinnabar is usually suspected. This work is focused on the blackening of red cinnabar paintings coated on a sparry calcite mortar. Different samples exhibiting different levels of degradation were selected upon visual observations and analyzed by synchrotron-based microanalytical techniques. Atomic and molecular compositions of the different debased regions revealed two possible degradation mechanisms. On one hand, micro X-ray fluorescence elemental maps show peculiar distributions of chlorine and sulfur. On the other hand, X-ray absorption spectroscopy performed at both Cl and S K-edges confirms the presence of characteristic degradation products: (i) Hg−Cl compounds (e.g., corderoite, calomel, and terlinguaite), which may result from the reaction with exogenous NaCl, in gray areas; (ii) gypsum, produced by the calcite sulfation, in black coatings. Metacinnabar is never detected. Finally, a cross section was analyzed to map the in-depth alteration gradient. Reduced and oxidized sulfur distributions reveal that the sulfated black coating consists of a ∼5-μm-thick layer covering intact cinnabar.