- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Structural biology
- Cellular water transport: sub-angstrom resolution insights from the crystal structure of a membrane protein
Cellular water transport: sub-angstrom resolution insights from the crystal structure of a membrane protein
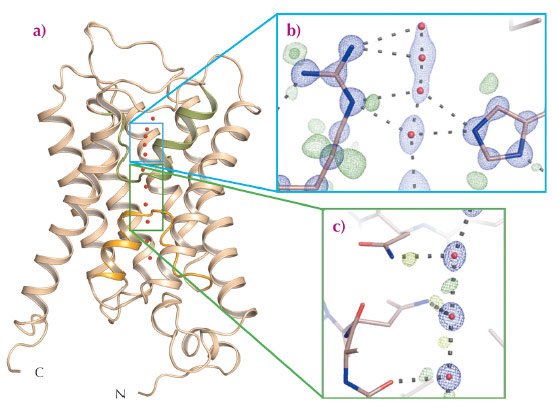
The control of water homeostasis in living cells is facilitated by aquaporins which, like all membrane proteins involved in homeostasis, have to be extremely selective for the molecule that they transport. Aquaporins must exclude both hydroxide (OH–) and hydronium (H3O+) ions as well as preventing the transport of protons (H+), which can usually be rapidly exchanged between water molecules linked by hydrogen bonds. The crystal structures of aquaporins from several species show that their tertiary structures comprise six transmembrane helices plus a pseudo helix, made up of two aligned half-helices inserted from opposite sides of the membrane, arranged to form a pore through which water can flow (Figure 45a). The positioning of the pseudo helix places the dual asparagine-proline-alanine (NPA) motif characteristic of aquaporins close to the centre of the pore (Figure 45c), with water specificity supplied by a so-called ‘selectivity filter’ found close to the extracellular pore entrance (Figure 45b). The two half-helices making up the pseudo transmembrane helix focus a positively charged electrostatic dipole at the centre of the channel and this has been proposed to create a barrier preventing the passage of protons, and also to orient the dipoles of water molecules passing though the pore in a way that prevents proton exchange between water molecules via a Grotthuss mechanism [1, 2]. This hypothesis, however, does not explain the observation that mutations that reduce the strength of this electrostatic barrier increase the transport of Na+ by aquaporins but not that of H+ [3].
 |
|
Fig. 45: Sub-angstrom resolution crystal structure of an aquaporin isolated from yeast. a) The fold of an aquaporin monomer comprises six transmembrane helices and one pseudo-transmembrane helix (formed by two aligned half helices, shown in orange and green) arranged to form a pore through which water can flow. b) Electron density for the selectivity filter region of the aquaporin structure solved to 0.88 Å resolution. Blue mesh shows the 2mFobs-DFcalc electron density around the selectivity filter residues Arg227 and His212, as well as around four water molecules identified within the selectivity filter (red spheres). Green mesh represents residual mFobs-DFcalc electron density, indicating the presence of electrons associated with hydrogen bonds or involved in covalent bonds between hydrogen and carbon atoms. c) Electron density near the dual asparagine-proline-alanine (NPA) aquaporin signature motif. Blue mesh represents 2mFobs-DFcalc electron density for water molecules (red spheres) near the centre of the aquaporin pore; green mesh reveals electron density for electrons participating in hydrogen bonds with water molecules. H-bond donor interactions from the nitrogen atoms of the two NPA motif alanine residues (Ala112 and Asn224) to passing water molecules are clearly observed. |
The ultra-high resolution (dmin = 0.88 Å) crystal structure of Aqy1 from Pichia pastoris, obtained using data collected at beamline ID29, has shed new light on the selectivity mechanisms of water transport by aquaporins. The electron density maps allow the assignment of H-bond interactions of both NPA asparagine residues to passing water molecules (Figure 45c) and confirm a proposed bipolar distribution of hydrogen bonds between adjacent water molecules within the aquaporin channel. Similarly, both the orientation and tautomeric forms of two conserved selectivity filter residues, His212 and Arg227, can be assigned (Figure 45b). Intriguingly, the electron density also revealed stable sites for four water molecules within the selectivity filter (Figure 45b). However, the distances between these water positions excludes that all four sites are occupied simultaneously and, in fact, these four positions are made up of two pairs of sites that are partially occupied in a mutually exclusive fashion (i.e. sites Wat2 and Wat4 cannot be occupied at the same time as sites Wat1 and Wat3). This suggests a pair-wise movement of water molecules through the aquaporin selectivity filter, analogous to the crystallographically observed pair-wise transport of K+ through potassium channels [4]. As was proposed for potassium transport, the similar occupancy of the four water sites (66% for Wat2 and Wat4; 34% for Wat1 and Wat3) suggests that the binding energies of these pairs are much the same, a situation which is ideal for maximising water conduction rates in aquaporins.
Principal publication and authors
U. Kosinska Eriksson (a), G. Fischer (a), R. Friemann (a), G. Enkavi (b), E. Tajkhorshid (b) and R. Neutze (a), Science 340, 1346-1349 (2013).
(a) Department of Chemistry and Molecular Biology, University of Gothenburg (Sweden)
(b) Department of Biochemistry, College of Medicine, Center for Biophysics and Computational Biology, and Beckman Institute for Advanced Science and Technology, University of Illinois, Urbana (USA)
References
[1] E. Tajkhorshid et al., Science 296, 525-530 (2002).
[2]. B.L. de Groot, T. Frigato, V. Helms and H. Grubmüller, J. Mol. Biol. 333, 279-293 (2003).
[3] D. Wree, B. Wu, T. Zeuthen and E. Beitz, FEBS J. 278, 740-748 (2011).
[4] J.H. Morais-Cabral, Y. Zhou and R. MacKinnon, Nature 414, 37-42 (2001).



