- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structural biology
- Insight into an intermediate step in O2-dependent catalysis
Insight into an intermediate step in O2-dependent catalysis
The unambiguous identification of reaction intermediates is one of the most challenging tasks in structural enzymology. Although peroxide intermediates are likely to be formed in many reactions involving molecular oxygen (O2), their structural identification has so far proven elusive. Here, X-ray crystallography combined with online in-crystallo Raman spectroscopy at beamline ID29 and radiation damage effects have provided direct evidence for a C5(S)-(hydro)peroxide intermediate in a cofactor-free oxidase reaction that also allowed the visualisation of the reactive enzyme-substrate-O2 complex.
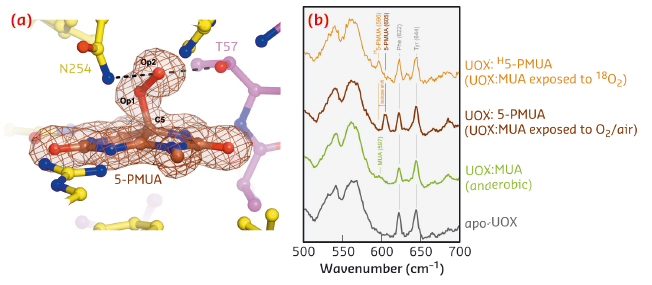
Uricase (UOX) is the archetypal cofactor-independent oxidase [1]. It catalyses the breakdown of uric acid to 5-hydroxyisourate. A recombinant version of UOX finds therapeutic application to aid uric acid clearance in paediatric and adult cancer patients and for gout treatment. To investigate the step involving dioxygen in UOX-mediated catalysis, we employed 9-methyl uric acid (MUA). When anaerobic UOX:MUA co-crystals are exposed to O2, electron density maps at 1.3 Å resolution unambiguously reveal that MUA converts into its C5(S)-peroxo derivative (5-PMUA) (Figure 114a). 5-PMUA is sp3 hybridised at carbon C5. The peroxo Op1-Op2 bond is 1.47 Å long, whilst the length of the C5-Op1 bond refines to 1.51 Å.
Non-resonant Raman spectra recorded from crystals of substrate-free UOX, anaerobic UOX:MUA and UOX:5-PMUA complexes are very similar overall. However, the region centred around 600 cm-1 exhibits changes sensitive to the chemistry of the oxygenation reaction (Figure 114b). Upon MUA peroxidation, a distinct band develops at 605 cm-1 (brown trace), whilst the shoulder at 597 cm-1 for the UOX:MUA complex (green) disappears. Neither band is present in the spectrum of substrate-free UOX (grey) indicating that they arise from the bound organic molecules. Furthermore, the 605 cm-1 band could be selectively shifted by carrying out MUA peroxidation with 18O2 (shift to 596 cm-1, Δ~![]() = –9 cm-1, gold), thus confirming that this band specifically involves Raman modes with contributions from the peroxide oxygen atoms. Quantum mechanics/molecular mechanics (QM/MM) calculations at the MP2/6-31+G* level of theory predict a band at 600 cm-1 (experimental 605 cm-1) for the 5-PMUA hydroperoxide resulting from a set of modes involving C5-Op1 bond stretching and C5-Op1-Op2 bending coupled to ring distortions. The theory further predicts a -9 cm-1 isotope shift (experimental -9 cm-1) for 5-PMUA featuring 18Op1 and 18Op2 peroxide oxygen atoms. Overall, the calculations agree remarkably well with the experiment identifying the 605 cm-1 5-PMUA Raman band as a ‘signature’ for its peroxide state.
= –9 cm-1, gold), thus confirming that this band specifically involves Raman modes with contributions from the peroxide oxygen atoms. Quantum mechanics/molecular mechanics (QM/MM) calculations at the MP2/6-31+G* level of theory predict a band at 600 cm-1 (experimental 605 cm-1) for the 5-PMUA hydroperoxide resulting from a set of modes involving C5-Op1 bond stretching and C5-Op1-Op2 bending coupled to ring distortions. The theory further predicts a -9 cm-1 isotope shift (experimental -9 cm-1) for 5-PMUA featuring 18Op1 and 18Op2 peroxide oxygen atoms. Overall, the calculations agree remarkably well with the experiment identifying the 605 cm-1 5-PMUA Raman band as a ‘signature’ for its peroxide state.
 |
|
Fig. 114: a) The UOX: 5-PMUA complex formed by reacting UOX:MUA crystals grown in anaerobic conditions with air. Electron density is shown for 5-PMUA. Hydrogen bonds involving the peroxo atoms are shown by broken lines. b) In-crystallo Raman spectra of apo-UOX (grey), anaerobic UOX:MUA (green), UOX:5-PMUA (brown), and UOX:H5-PMUA (gold). H5-PMUA (H for heavy) refers to MUA reacted with 18O2. |
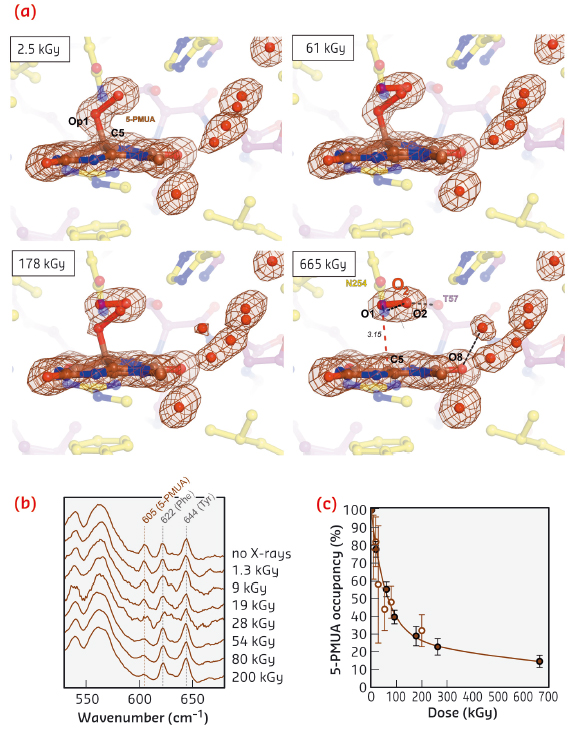
Exposure to X-rays can lead to specific modifications in proteins and nucleic acids, including bond rupture. We observed that the C5-Op1 bond is susceptible to selective radiolysis at very low X-ray doses. We tracked this quantitatively using on line Raman-assisted crystallography by performing multiple data collections interspersed by spectrophotometric measurements on a single UOX:5-PMUA crystal. The dose-dependent rupture of the C5-Op1 bond is accompanied by the loss of Sp3 hybridisation at C5 leading to a planar organic structure (Figure 115a 2.5-655 kGy). Concomitantly, a diatomic molecule with a shorter bond length (1.2 Å) than the original peroxide is liberated and trapped above it. We interpreted the elongated electron density as molecular oxygen as reactive oxygen species are likely transiently formed in the process and are expected to rapidly convert to the more stable O2 molecule. Dioxygen adopts a well-defined position and orientation above the flat organic molecule. The O2 molecular axis lies parallel to the plane at a distance of 3.15 Å and is rotated by 15° as compared to the original Op1-Op2 peroxide bond (Figure 115a).
 |
|
Fig. 115: a) Dose-stamped snapshots for UOX:5-PMUA radiolysis. Electron density is shown for the organic moieties and solvent molecules in close proximity. b) Online in-crystallo Raman spectroscopy reveals the specific dose-dependent decrease of the 605 cm-1 5-PMUA ‘fingerprint band’. c) 5-PMUA decay is biphasic suggesting a mechanism of peroxide regeneration. 5-PMUA occupancies from crystallographic refinement are shown as filled circles. Open circles are 5-PMUA occupancies estimated from the integration of the 605 cm-1 Raman band. |
Online Raman analysis shows that peroxide rupture causes the height of the 605 cm-1 ‘signature band’ to selectively decrease in a dose-dependent manner (Figure 115b), consistent with our QM/MM calculations that assign this band to the stretching of the C5-Op1 bond. The dose-dependent 5-PMUA occupancy from crystallographic refinement at near-atomic resolution is in excellent agreement with its orthogonal estimation from the integration of the 605 cm-1 Raman signal (Figure 115c). 5-PMUA decay follows a biphasic profile. This is not consistent with a simple peroxide decomposition process. The observed decay can be rationalised assuming a pathway of peroxide regeneration acting alongside its decomposition. We have fitted this process kinetically.
Our multi-technique investigation offers unambiguous evidence for a C5-peroxide intermediate in cofactor-free UOX catalysis. Peroxide radiolysis followed by online Raman-assisted X-ray crystallography afforded exquisite insight into the elusive reactants’ configuration leading to the peroxo intermediate. The latter is suggested to be formed by a radical recombination process.
Principal publication and authors
S. Bui (a), D. von Stetten (b), P.G. Jambrina (c), T. Prangé (d), N. Colloc’h (e), D. De Sanctis (b), A. Royant (b,f), E. Rosta (c) and R.A. Steiner (a), Angew. Chem. Int. Ed. 53, 13710–13714 (2014).
(a) Randall Division of Cell and Molecular Biophysics, King’s College London (UK)
(b) ESRF
(c) Dept. of Chemistry, King’s College London (UK)
(d) LCRB, UMR 8015-Université Paris Descartes-CNRS (France)
(e) ISTCT, UMR 6301–UCBN–CNRS–CEA-Normandie Université (France)
(f) IBS, Grenoble (France)
References
[1] S. Fetzner and R.A. Steiner, Appl. Microbiol. Biotechnol. 86, 791-804 (2010).



