- Home
- News
- Spotlight on Science
- Real-time detection...
Real-time detection of molecules secreted from single cells stimulated by an X-ray nanobeam
31-08-2020
The molecular release from living cells stimulated by high-spatial-resolution X-ray irradiation was observed in vitro for the first time. The phenomenon was investigated at the single-cell level by exploiting a diamond-based biosensor in combination with an X-ray nanobeam.
Ionising radiation is an effective tool employed in cancer therapy but at the same time, it is a cause of secondary cancer. For these reasons, a large number of previous studies was focused on the investigation of the effects induced in living systems by the exposure of ionising radiation at non-lethal doses. In particular, unexpected consequences such as bystander (biological response of a cell resulting from an event in an adjacent or nearby cell) or abscopal effects (regression of non-irradiated metastatic lesions) were observed, but several questions related to the mechanism associated with these phenomena are widely debated and still open. New experimental approaches are essential to shed light on these questions, especially in the study of the effects induced at the neuronal level, which are mostly unknown.
In this context, the release of molecules from cells triggered by X-ray irradiation was observed for the first time through an experiment at beamline ID16B. In particular, a real-time amperometric detection of secreted neurotransmitters from a single neuroendocrine cell was performed.
Today, the combination of radiotherapy with immunotherapy has a growing consensus, providing an opportunity for improving the efficacy of the abscopal response, extending the use of radiotherapy to the metastatic disease. Therefore, radiation-induced secretion represents an unexplored effect which can be studied to define effective tumour treatments.
In this study, the PC12 cell line was employed, which was derived from pheochromocytoma (a tumour) of the rat adrenal medulla that has been immortalised to proliferate indefinitely. This cell line presents secretion of molecules mediated by vesicles (exocytosis) containing catecholamines, mostly dopamine and a limited amount of norepinephrine [1].
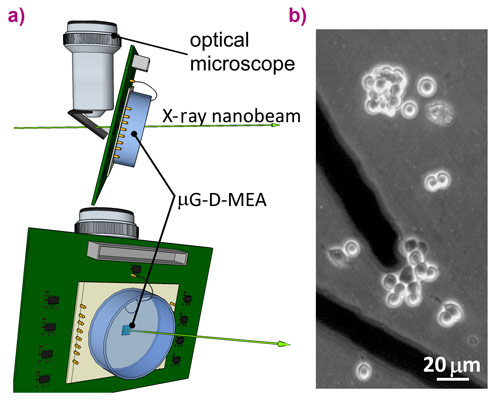
The cellular exocytotic activity was monitored using diamond-based biosensors consisting of a single-crystal substrate equipped with a multi-electrode array of graphitic microchannels guaranteeing the parallel detection of biosignals from individual cells [2,3]. Moreover, the sensors also allow the simultaneous detection of the photocurrent generated by the passage of the X-ray beam that was employed to synchronise the X-ray delivery starting time with the amperometric recording during the single-cell irradiation.
During the experiment, the same electronic chain was used to detect both the dosimetric and the biophysical signals since the induced photocurrent and the biosignals ascribable to exocytosis events are characterised by different time-scales and therefore were simultaneously recorded in single chronoamperograms.
Irradiation was carried out using an in-air focused X-ray beam with energy of 17.4 keV and spot size of 55 nm × 60 nm. The biosensor, properly connected with the front-end electronics, was placed in the focal plane of the X-ray beam, which corresponds to those of a conventional optical microscope allowing sample observation during the experiment (Figure 1). The PC12 cell line was plated directly over the diamond-based sensor.
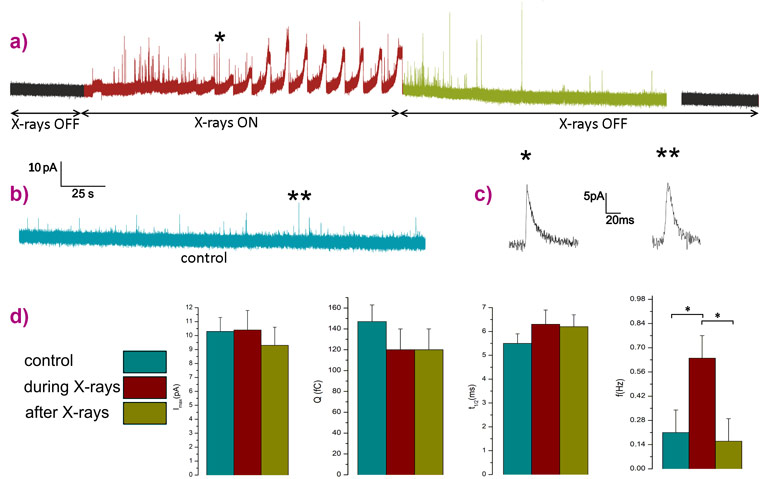
The irradiations were performed by targeting single cells with a flux of 7×108 photons s-1, a dose low enough to keep the cells alive while simultaneously detecting both the photocurrent and the exocytotic signals. The chronoamperogram reported in Figure 2 has two regions of interest highlighted in red and green. In red, the region with X-rays on is characterised by broad and intense peaks associated with the detection of the photocurrent which are overlapped by many amperometric spikes indicating the stimulation of an intense exocytotic pattern after starting the raster scanning of the X-ray beam across a PC12 cell that was initially silent. In green, the region after switching off the X-rays, the secretory activity is still present for a few minutes and then becomes undetectable.
The kinetics (full-width half-maximum) and intensity parameters (maximum peak current and quantal charge) of the amperometric spikes detected from both irradiated cells and control cultures (PC12 cell line plated using the same protocol but not irradiated) were analysed. The values obtained are statistically compatible revealing that the secretory pathway remained unaltered in the X-ray treated cells. Meanwhile, the frequency parameter (number of spikes per unit of time) is characterised by a significant increment during the irradiation representing direct evidence that X-rays selectively stimulate the exocytotic activity.
In conclusion, the combined use of diamond-based multi-electrode biosensors and synchrotron nanometric X-ray beams enabled the study at the single-cell level of the effect of ionising radiation by recording secretory activity in real-time while simultaneously monitoring the delivered beam. This approach opens new perspectives in experimental radiobiology for the investigation of radiation effects on specific organelles with sub-micrometric spatial resolution. Moreover, the observation of the X-ray stimulation of dopamine release represents the first demonstration of a novel effect that could potentially have great implications for cancer treatment by radiotherapy.
Principal publication and authors
F. Picollo (a,b), G. Tomagra (a), V. Bonino (a,b), V. Carabelli (a), L. Mino (a), P. Olivero (a,b), A. Pasquarelli (c), M. Truccato (a,b), Triggering neurotransmitters secretion from single cells by X‑ray nanobeam irradiation, NanoLetters 20, 3889 (2020); doi: 10.1021/acs.nanolett.0c01046.
(a) University of Torino (Italy)
(b) National Institute of Nuclear Physics - INFN, Torino (Italy)
(c) University of Ulm (Germany)
References
[1] Tomagra G. et al., Biophysical Chemistry 253106241 (2019).
[2] Picollo F. et al., Advanced Materials 25, 4696 (2013).
[3] Tomagra G. et al., Carbon 152, 424 (2019).