- Home
- News
- Spotlight on Science
- High-pressure synthesis...
High-pressure synthesis of phosphine from the elements and the discovery of the missing (PH3)2H2 tile
01-12-2020
A new inorganic route for the synthesis of phosphine from black phosphorus and molecular hydrogen, and a crystalline van der Waals compound never observed before for any group 15 element, have been discovered under high-pressure and -temperature conditions at beamline ID27.
Using synchrotron X-ray diffraction (XRD) at beamline ID27 and infrared and Raman spectroscopy at LENS in Italy, it has been demonstrated that black phosphorus (bP) and molecular hydrogen (H2) directly react to form phosphine (PH3) under high-pressure and high-temperature conditions (P=1.2 GPa, T≲1000 K) generated by means of a diamond anvil cell (DAC) and laser-heating.
To perform this experiment, a small piece of high purity bP, synthesised at ICCOM-CNR in Italy, was placed in a DAC, which was then gas-loaded with H2. The sample was compressed to 1.2 GPa and laser-heated up to the occurrence of chemical reactivity, which was first visually detected by the change of the sample aspect using an optical microscope, and then clearly demonstrated by the disappearance of the Bragg peaks of bP in the diffractogram of the sample acquired after laser-heating. The formation of PH3 was confirmed by the observation of its characteristic vibrational signatures in the FTIR and Raman spectra.
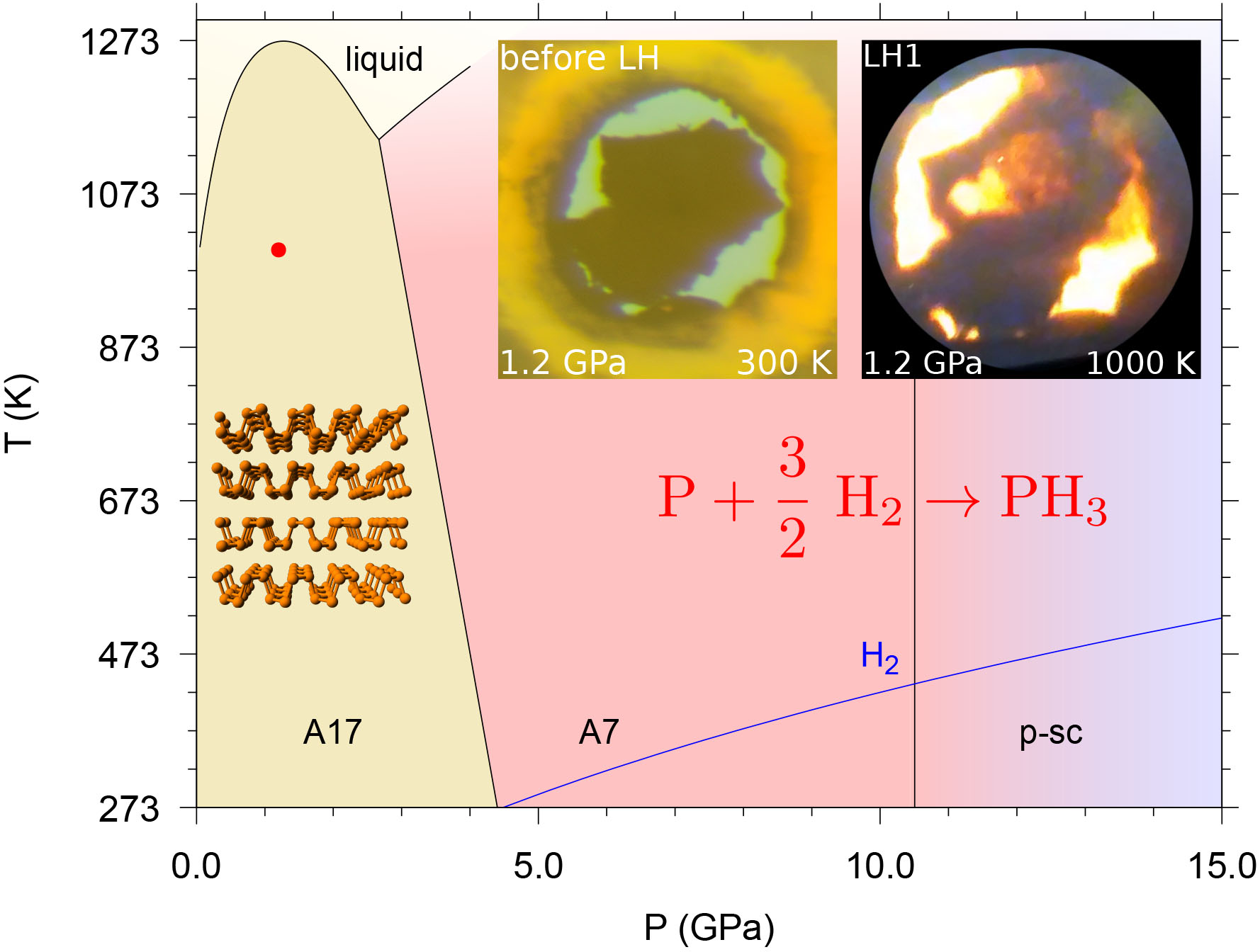
Fig. 1: Phase diagram of phosphorus (black lines), showing the stability regions of the orthorhombic (A17, bP), rhombohedral (A7) and pseudo simple-cubic (p-sc) [1,2] structures. The melting line of H2 (blue line) and laser-heating conditions (red point at P=1.2 GPa, T≈1000 K) are also displayed. The microphotographs in the top right corner show the sample aspect at 1.2 GPa before and during the first laser-heating irradiation, illustrating the transformation of phosphorus.
It was also discovered that at higher pressure, PH3 and H2 form a crystalline van der Waals (vdW) compound, (PH3)2H2. A single-crystal XRD pattern suddenly appears when further compressing PH3 and excess H2 between 3.5 and 4.1 GPa at room T. The analysis of the data indicates that the observed pattern corresponds to a tetragonal structure of P atoms, not compatible with any known structure of phosphorus and not explainable with the presence of PH3 only. Indeed, the appearance of one extra band in the H-H stretching region of the IR and Raman spectra at lower frequency with respect to pure H2, disappearing on decompression below the pressure threshold at which the crystallisation occurs, indicates the presence in the sample of two types of H2 molecules experiencing different interactions with the local environment.
Another key piece of information emerging from the Raman mapping of the sample is that the appearance of the low-frequency H-H stretching band is always associated with the presence of the bands of PH3, whereas it is not observed in regions of the sample where PH3 is missing, thus establishing a clear correlation between both spectroscopic signatures. A possible explanation for this occurrence, which is fully accounted for by the analysis of the spectroscopic activity of the Davydov components using group theory arguments in agreement with the single-crystal XRD data, is the formation of a crystalline vdW compound made of PH3 and H2 molecules, having a I4cm tetragonal structure, where PH3 and H2 respectively occupy 8c (Cs) and 4a (C4) Wyckoff sites. Within this clathrate-like structure, each H2 molecule is caged by 8 PH3 molecules. As no H2-containing vdW compound involving the molecular hydrides of a non-metallic element has been reported so far for any of the group 15 elements, the observation of (PH3)2H2 represents the missing tile for pnictogens in the periodic table.
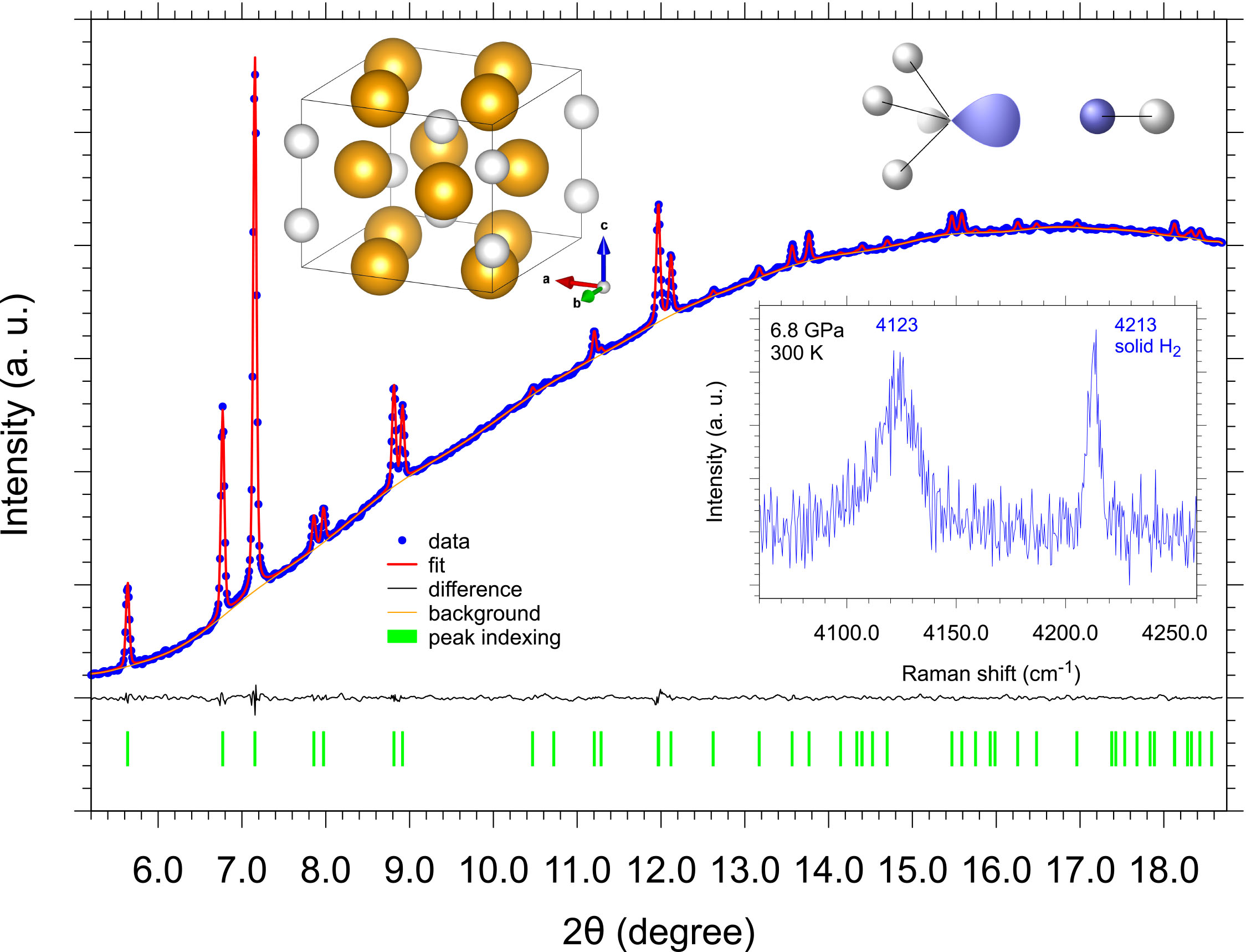
Fig. 2: Integrated panoramic XRD pattern of the (PH3)2H2 crystalline vdW compound acquired after laser-heating of bP and H2 at 1.2-1.5 GPa (T≲1000 K) and room T compression to 5.5 GPa (ID27, λ= 0.3738 Å). The Le Bail fit, represented by the red trace, is based on single-crystal data refinement, whose resulting I4cm structure is shown in the top part of the figure, with the orange and white spheres respectively representing PH3 and H2 molecules. The extra Raman band, observed in the H-H stretching region at lower frequency with respect to pure H2, and assigned to H2 molecules forming the (PH3)2H2 crystalline vdW compound, is shown in the bottom right corner of the figure. The drawing in the top right corner of the figure illustrates the molecular orbital interaction between the HOMO of PH3 and the LUMO of H2.
The results of this study have multiple relevant implications. First, besides addressing fundamental issues related to the chemistry of the elements under extreme conditions, the direct uncatalysed reactivity between bP and H2, leading to the synthesis of PH3, represents the as-yet unreported P analogue of the famous Haber-Bosch reaction, in which NH3 is synthesised from N2 and H2.
Second, the discovery of the (PH3)2H2 vdW compound, never before observed for any of the group 15 elements, not only represents the missing tile of pnictogens, specifically of P, in the periodic properties of non-metallic elements that are able to form H2-containing vdW compounds of their hydrides, but also confirms a general trend from group 14 to group 17 in the formation of vdW compounds having X2H2 stoichiometry, also shedding new light on the debated structure of (H2S)2H2 phase I, which is of extreme interest as a starting material for the synthesis of high-temperature superconducting materials at high pressure.
Third, the orbital interaction between the highest occupied molecular orbital (HOMO) of PH3 and the antibonding lowest unoccupied molecular orbital (LUMO) of H2, which is proposed to stabilise the (PH3)2H2 vdW compound, besides being relevant to bond theory, once again confirms the key role played in the high-pressure behaviour of P by the electron lone pair typical of its sp3 hybridisation [1,2], as also supported here by the evidence of H-bonding between PH3 molecules, not observed at ambient pressure. Such interaction provides new experimental insight, possibly helping to understand the effects underlying the predicted stabilisation of the P-H systems under high-pressure conditions. (PH3)2H2 represents the first confirmatory experimental evidence for the key role played by H2 molecules in stabilising the P-H system at high pressure, as suggested by recent calculations predicting the presence of H2 units in superconducting P-H structures at high pressure.
Finally, the synthesis of PH3 from the elements and its interaction with H2 are of importance for energetic issues related to hydrogen storage and conversion, and for astrochemical processes occurring in extra-terrestrial environments of giant planets such as Jupiter, Saturn and their moons. As recently emerged with the claim of the possible presence of life on Venus from the detection of anomalously high levels of phosphine in the cloud decks of Venus’ atmosphere, the identification of any inorganic chemical route to the synthesis of phosphine in the harsh conditions of rocky planets has indeed become relevant.
Principal publication and authors
High pressure synthesis of phosphine from the elements and the discovery of the missing (PH3)2H2 tile, M. Ceppatelli (a,b), D. Scelta (a,b), M. Serrano-Ruiz (b), K. Dziubek (a,b), G. Garbarino (c), J. Jacobs (c), M. Mezouar (c), R. Bini (a,b,d), M.Peruzzini (b), Nat. Commun. (2020); https://doi.org/10.1038/s41467-020-19745-2.
(a) LENS, European Laboratory for Non-linear Spectroscopy, Firenze (Italy)
(b) ICCOM-CNR, Institute of Chemistry of OrganoMetallic Compounds, National Research Council of Italy, Firenze (Italy
(c) ESRF
(d) Dipartimento di Chimica “Ugo Schiff” dell’Università degli Studi di Firenze, Firenze (Italy)
References
[1] D. Scelta et al., Angew. Chem. Int. Ed. 56, 14135 (2017).
[2] D. Scelta et al., Chem. Commun. 54, 10554 (2018).



