- Home
- News
- Spotlight on Science
- An unexpected new...
An unexpected new cubic symmetry in group IV alloys
22-02-2021
Researchers have synthesised a new cubic group IVA alloy, Ge-Sn, a material actively investigated for optoelectronic applications to overcome conventional cubic Si’s deficiencies. Using high-pressure, high-temperature experiments, reactivity barriers between Ge and Sn were removed. For this, conventional routes to synthesis were neither followed nor required.
Share
The group IVA column of elements (C, Si, Ge, Sn, Pb) occupies a prominent position in the periodic table. The cubic diamond symmetry (Fd-3m) is a prominent contributor to this position, as exemplified by super-hard cubic C and through cubic Si, the material driver behind the electronics industry. Using high-pressure, high-temperature multi-anvil experiments coupled with in-situ angle-dispersive X-ray diffraction measurements at beamline ID06-LVP, researchers have synthesised and characterised an unexpected new cubic group IVA alloy, namely body centred cubic (Im-3m) Ge-Sn.
Ge-Sn itself is one of the most actively investigated systems for direct band gap formation as opposed to Si’s indirect band-gap [1,2]. However, Ge and Sn do not react in the bulk at ambient pressure; hence, investigations have been largely confined to thin films. In this work, a Ge-Sn solid solution was synthesised upon heating Ge and Sn at pressures from 13 to 28 GPa. This was done using multi-anvil methods at beamline ID06-LVP and double‐sided diamond anvil laser‐heating at DESY, both characterised in situ with angle-dispersive X-ray diffraction measurements, making it possible to access regions in the new phase diagram where barriers to reactivity were removed. The new material substantially enriches the seminal group IVA alloy materials landscape by introducing an eightfold coordinated cubic symmetry (Im-3m) (Figure 1), which markedly expands on the conventional tetrahedrally coordinated cubic one (Fd-3m).
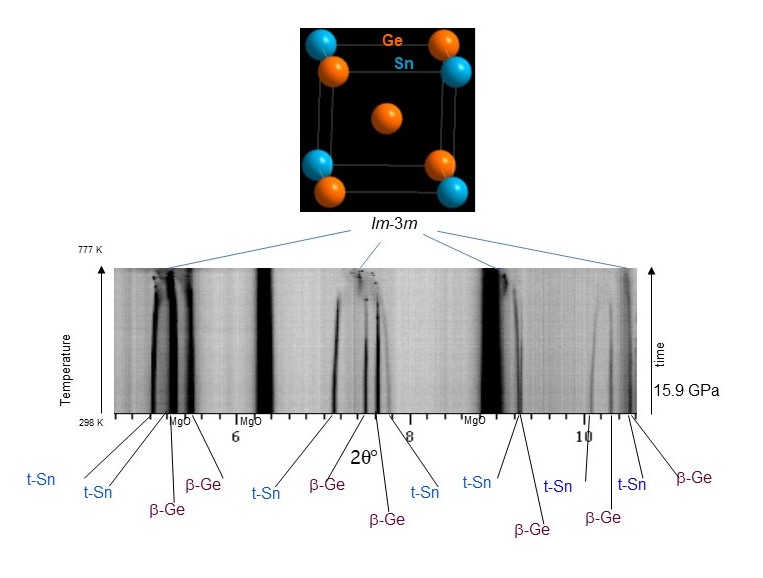
Fig. 1: Detailed evolution of angle-dispersive X-ray diffraction patterns at ID06-LVP upon heating of a β-Ge (I41/amd) and t-Sn (I4/mmm) mixture at 15.9 GPa in a multi-anvil device and formation of a bcc structure with Im-3m symmetry {a = 3.481 (1) Å} at 777 K (λ = 0.22542 Å). The MgO diffraction lines belong to a principal component of the high-pressure assembly.
There are several unusual aspects associated with the synthesis and structure of this new cubic group IVA solid solution. A first unusual aspect is that Ge and Sn have unlike crystal structures and significantly different atomic radii in the range of synthesis conditions of the work (13-28 GPa), attributes which should not warrant solid solution formation. A second unusual aspect is that Ge, specifically because of its more localised electronic structure, does not adopt the Im-3m symmetry under any conditions, and thus should hinder, not promote, alloy formation with this symmetry. However, upon alloying with Sn, solid solution formation is not hindered but enhanced, with the formation pressure of pure Im-3m lowered by a staggering 500 000 atmospheres from that at which elemental Sn can adopt this symmetry as a single phase. A third unusual aspect is that melting, normally an integral part of synthesis to promote homogenisation, reactivity and crystal formation, is detrimental here, in contrast to pure solid-state reaction, which leads to high-quality crystal formation.
The explanation for the first unusual aspect is that the atomic radii within the coordination polyhedra of the reactant elements are not the appropriate compatibility manometer here. Instead, it is the radii that the elements will adopt in the coordination polyhedra of the new solid solution (Figure 2). For the second unusual aspect, while stabilisation of the new solid solution is not favoured by the electronic structure of Ge, it is favoured by the entropic contribution of Ge. This stabilising effect will be further strengthened with increasing temperature via the TΔS term in the Gibbs free energy expression. Temperature also contributes favourably to product formation with respect to relative specific volumes of product to reactants. An explanation for the third unusual aspect is that the local Sn liquid structure is considerably more anisotropic than the underlying Sn crystal structure, at the same pressure. Hence on melting, templating to highly anisotropic local polyhedral units hinders quenching of the new isotropic cubic phase. This is in sharp contrast to interdiffusion upon heating purely in the solid state, where the structural environment is considerably less anisotropic. Solid-state reaction is also assisted by the low activation barrier for diffusion of Sn, where noticeable diffusion already starts as low as 391 K.
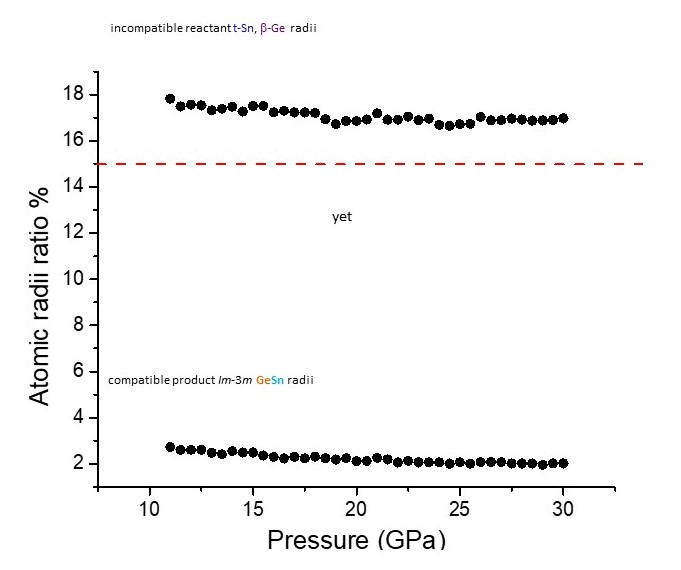
Fig. 2: Ge-Sn atomic radii ratio (%) pressure dependence {(Sn radius-Ge radius)/Sn radius} x 100. The red line is the ratio above which solid solutions are not favoured. The curve above the red line contains the atomic radii ratios (%) of the reactants t-Sn (I4/mmm) and β-Ge (I41/amd). The curve below the red line are the atomic radii ratios (%) of Sn and Ge in eightfold coordination, as present in the Im-3m structure.
The demonstration that this cubic solid solution is formed without adherence to conventional formation criteria and routes to synthesis creates new fertile avenues for exploring and exploiting new materials development including semimetals, post-transition metals and semiconductors. The new Ge-Sn cubic alloy, stable above 10 GPa, is a fountainhead for production on pressure release of a range of phases with optoelectronic potential currently being investigated. The emergence of this previously hidden group IVA alloy structure is a source for further enrichment of what is arguably the most technologically important column of the periodic table.
Principal publication and authors
An unexpected cubic symmetry in group IV alloys prepared using pressure and temperature, G. Serghiou (a), H.J. Reichmann (b), N. Odling (c), K. Spektor (d), A. Pakhomova (e), W. Crichton (d), Z. Konôpková (e), Angew. Chem. Int. Ed. (2021); https://doi.org/10.1002/anie.202016179
(a) School of Engineering, University of Edinburgh, Edinburgh (UK)
(b) Deutsches GeoforschungsZentrum, GFZ, Potsdam (Germany)
(c) School of Geosciences, University of Edinburgh, Edinburgh (UK)
(d) ESRF
(e) Deutsches Elektronen-Synchrotron, DESY, Hamburg (Germany)
References
[1] A. Elbaz et al., Nat. Photonics 14, 375-382 (2020).
[2] G. Serghiou et al., Inorg. Chem. 53, 5656-5662 (2014).



