- Home
- News
- Spotlight on Science
- Mycoplasmas use...
Mycoplasmas use molecular 'judo armlock' to evade host immune response
22-03-2021
The cryo-electron microscopy structure of mycoplasma MIB and MIP proteins in complex with a Fab, obtained at beamline CM01, reveals how these proteins capture and break down antibodies by performing a molecular ‘judo armlock’, shedding light on how mycoplasmas evade the host immune system.
Successful pathogens have developed multiple strategies to evade their host immune systems, in order to persist and propagate. Mycoplasmas are small bacteria able to colonise the mucosal surface of a wide range of hosts, including livestock species and humans. Many mycoplasma species are pathogenic and are naturally unresponsive to many antibiotics. The emergence of strains resistant to the few effective antibiotics (tetracycline in particular) is a cause for concern, in both the medical and veterinary fields. Most mycoplasmas are responsible for chronic respiratory, genital or urinary infections, suggesting that these bacteria are able to evade the host immune system. In particular, mycoplasmas need to escape antibody-mediated recognition and elude immune neutralisation and clearance.
Mycoplasmas have developed a two-protein system, referred to as the MIB-MIP system, based on Mycoplasma Immunoglobulin Binding (MIB) and Mycoplasma Immunoglobulin Protease (MIP), which respectively bind and cleave antibodies [1]. MIB recognises and captures the antibodies and subsequently recruits the serine protease MIP, which cuts off the antigen recognition domain VH, resulting in the inactivation of the antibodies.
A team of researchers from the CNRS at the IECB in Bordeaux, in collaboration with researchers from the University of Bordeaux and INRAE, have deciphered the mechanism of action of the MIB-MIP system, visualising its molecular architecture at the atomic scale using cryo-electron microscopy. They have solved the structure of the proteins MIB and MIP in complex with a goat IgG Fab (Figure 1) using the ESRF Titan Krios electron microscope at beamline CM01, with an overall resolution of 2.8 Å.
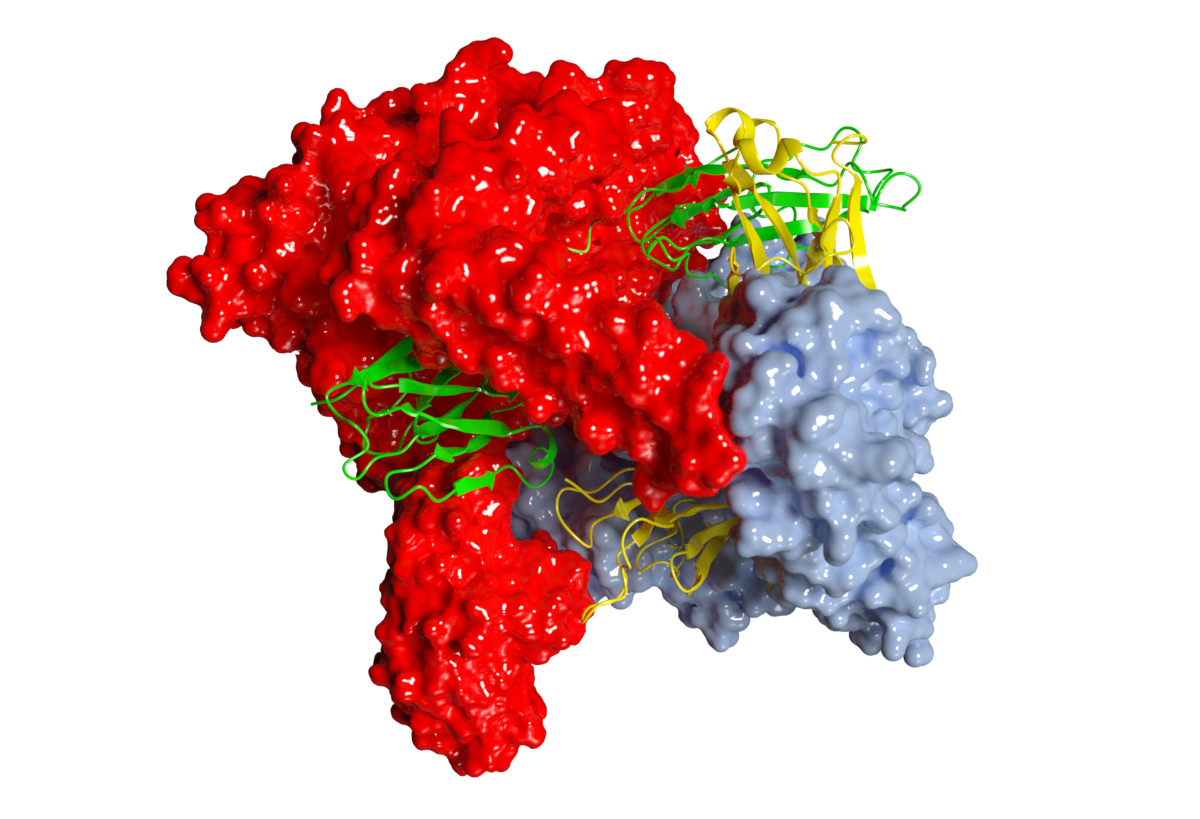
Fig. 1: Structure of MIB and MIP proteins in complex with Fab. MIB and MIP are represented as surfaces in blue and red respectively. The Fab is shown as ribbon representation with the light and heavy chains in green and yellow respectively.
The cryo-EM structure reveals a completely new mechanism, which resembles the "juji gatamé" armlock technique used in ju-jitsu or judo (Figure 2). MIB and MIP encircle the Fab fragment, holding it firmly in their arm domains. The linker between the antibody’s CH and VH domains is twisted and put in a favourable orientation to be cleaved by MIP. In addition, the conformation of the Fab in the MIB-MIP-Fab complex is drastically impaired. The VL and the VH fragments are twisted out of alignment and the antigen-binding site is torn apart. The distance between the VL and the VH complementary-determining regions (CDRs) is nearly twice the usual distance measured in an isolated Fab. These data reveal that the MIB-MIP complex, and MIB alone, are able to promote the dissociation of the antigen-antibody complex.
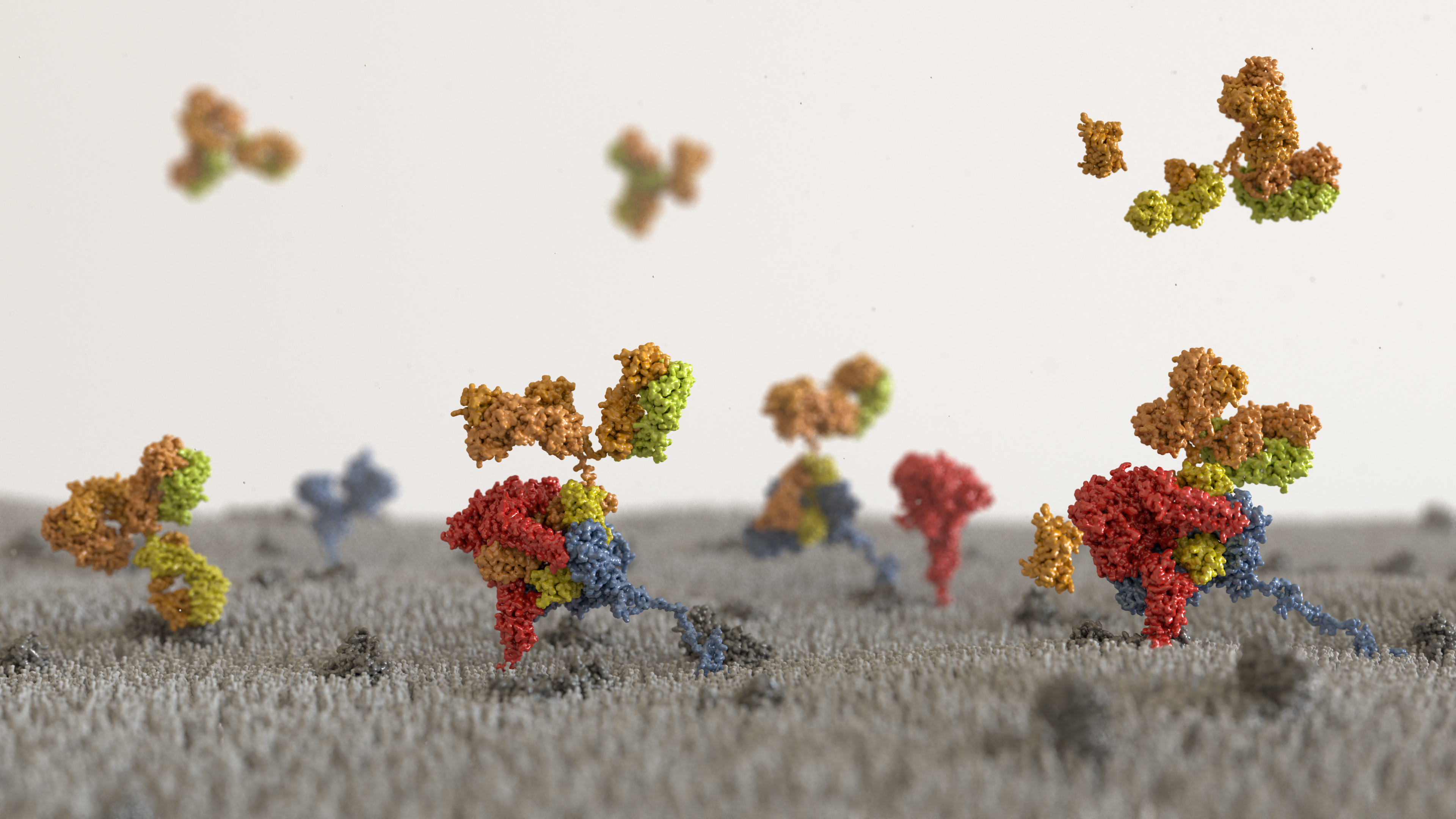
Fig. 2: Artistic representation of MIB and MIP in action at the surface of a mycoplasma cell. Atomic models of full length MIB and MIP are represented as surfaces in blue and red respectively. Immunoglobulins are represented as surfaces with their light chain in yellow and their heavy chains in orange. First, an immunoglobulin bound to a mycoplasma surface antigen is recognised by MIB and displaced from this antigen. The VH domain of the immunoglobulin is shifted away from its initial position freeing the linker between the CH and VH domains of the Fab. Subsequently, the protease MIP is recruited, the VH domain is cleaved off, and finally the cleaved antibody released from the cell surface.
These high-resolution data show that MIB and MIP use an unprecedented mechanism to dissociate antigen-antibody complex and capture and inactivate the antibody by proteolytic cleavage. They highlight the key role of MIB and MIP in the host immunity evasion by mycoplasmas, and indicate that MIB and MIP would be therapeutic targets of interest in veterinary and human health.
In addition to their relevance to the field of host-pathogen interaction, MIB and MIP could also become important biotechnology tools. The ability of MIB to promote the dissociation of the antibody-antigen complexes is unique. This property opens exciting perspectives to develop new biotechnological tools in the immunoglobulin field to manipulate antibodies or to dissociate antibodies bound to their antigen.
Principal publication and authors
The mycoplasma surface proteins MIB and MIP promote the dissociation of the antibody-antigen interaction, P. Nottelet (a), L. Bataille (b), G. Gourgues (b), R. Anger (a), C. Lartigue (b), P. Sirand-Pugnet (b), E. Marza (a), R. Fronzes (a), Y. Arfi (b), Sci. Adv. 7, 10 (2021); https://doi.org/10.1126/sciadv.abf2403
(a) Structure and Function of Bacterial Nanomachines, UMR 5234, Univ. Bordeaux, CNRS, Institut Européen de Chimie et Biologie, Pessac (France).
(b) Univ. Bordeaux, INRAE, Biologie du Fruit et Pathologie, UMR 1332, Villenave d’Ornon (France).
References
[1] Y. Arfi et al., Proc. Natl. Acad. Sci. U.S.A. 113, 5406-5411 (2016).



