- Home
- News
- General News
- The structure of...
The structure of a key enzyme for infectious diseases solved at ESRF
03-04-2003
A European team of scientists from the University of Dundee (UK), the Technical University of Munich (Germany) and the European Synchrotron Radiation Facility, ESRF1, (France) have determined the structure of a key target enzyme for novel drug development to treat infectious diseases including malaria, tuberculosis and sexually transmitted bacterial infections. The results of their collaboration are published on the August 5 issue of Proceedings of the National Academy of Sciences.
Share
|
Synchrotron radiation at the ESRF played a key role in the determination of the structure of the enzyme CDP-ME kinase. The experiment took place on one of the macromolecular crystallography beamlines at the ESRF. This kinase helps to produce many of the molecules that bacteria and parasites need to live and multiply. A molecule that can prevent the kinase from working normally will poison and kill the pathogenic organisms. The determination of the structure of the enzyme provides a template for the design of small molecules that will inhibit its action and prevent it from working normally. In the future, the structure may help lead to the development of new potent therapies for a wide range of microbial infections. These drugs could potentially help the treatment of not only malaria and tuberculosis but also toxoplasmosis, chlamydia, meningitis and cholera for example.
|
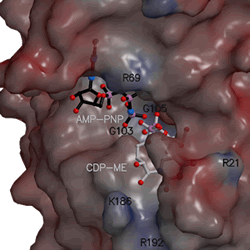
the molecular structure and shape of CDP-ME kinase |
|
The shape of the CDP-ME kinase active site with location of substrates. (full size .gif image - 165 kb)
|
About the technique
Protein crystallography requires the production of highly pure protein samples. These samples are then crystallised to produce single crystals of the protein. Interaction between the constituents of the crystal and X-rays results in the production of an interference pattern known as a diffraction pattern. With the help of specialised computer programs it is possible, starting from measurement of diffraction patterns, to determine the spatial distribution of the electrons within the crystals. Chemical interpretation of this produces an atomic model that reveals the three-dimensional structure of the protein molecules contained within the crystal.
The ESRF presents substantial advantages for the exploitation of this technique; in particular its intense, highly collimated X-ray beams greatly aid the investigation of small (or) weakly diffracting crystals.
For further information, please contact Montserrat Capellas, ESRF press officer, tel. +33 476 88 26 63 (email).
1The European Synchrotron Radiation Facility (ESRF) is constituted as an international facility with 17 participating countries to operate, maintain and develop a synchrotron radiation source and associated instruments. It operates the most powerful third generation synchrotron radiation source in Europe (www.esrf.fr).
How the synchrotron works
Electrons emitted by an electron gun are first accelerated in a linear accelerator (linac) and then transmitted to a circular accelerator (booster synchrotron) where they are accelerated to reach an energy level of 6 billion electron-volts (6 GeV). These high-energy electrons are then injected into a large storage ring -844 metres in circumference- where they circulate in a vacuum environment, at a constant energy, for many hours.
The synchrotron beams emitted by the electrons are directed towards the "beamlines" which surround the storage ring in the experimental hall. Each beamline is designed for use with a specific technique or for a specific type of research. Experiments run throughout day and night.