- Home
- News
- General News
- Transport protein...
Transport protein unravelled in two states
10-09-2010
Transport proteins carry molecules in the cell, but in some diseases, such as cystic fibrosis, they fail to do so. A team from the Max-Planck Institute for Biophysics in Frankfurt (Germany) has gained insight in a carnitine transporter, which, when faulty, causes the incurable Crohn’s disease. Their research is published this week in Nature.
Share
The team presents the structure of the carnitine transporter CaiT in two states. CaiT takes a nutrient molecule from the environment (in the case of the bacterium) or the gut (in the case of the human homologue) and pushes it through the diffusion barrier of the cell membrane. In exchange, it expels the metabolic product of carnitine (gamma-butyrobetaine) from the cell as a waste product. E. coli (or human) cells derive energy from metabolising carnitine.
“It is the first time that we could show that the activity of a transporter of this type is allosterically regulated by its own substrate. There are very few similar transporter structures in the Protein Data Bank and one big aim in the field is to understand the detailed transport mechanism. By comparing several structures in different states, the molecular mechanism becomes clear. We have determined not one, but two different new states of this type of transporter, so there is now, for the first time, a full picture of all stages of the transport mechanism,” explains Werner Kühlbrandt, leader of the team and director at the Max-Planck Institute for Biophysics.
 |
|
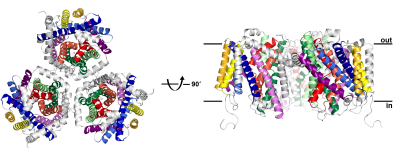
Structure of the carnitine transporter CaiT, determined at 2.3 Å resolution using synchrotron radiation at ESRF and SLS. CaiT is a membrane transport protein that forms trimers of three identical monomers in the cell membrane. Each protomer consists of 12 membrane-spanning alpha helices, including a core of 10 helices (coloured) that is conserved in other, structurally related secondary transporters. The CaiT structure was determined in two different states, with and without bound substrate molecules, indicating a new, allosteric mechanism of substrate transport across the membrane. Credits: W. Kühlbrandt. |
The research is still far from application, but it provides the scientific community some basic knowledge on this type of membrane protein. Synchrotron sources have helped the team to unveil these structures, and, in addition to the ESRF, they have also done measurements at the Swiss Light Source. “With more brilliant synchrotron beams, smaller crystals can be examined, and the advent of robotic sample changers has made it much easier to screen large numbers of crystals. Both are essential for membrane proteins, where crystals tend to be very small and diffract weakly,” explains Kühlbrandt.
Reference:
S Schulze et al. 2010 Nature 467 233–236.



